Abstract
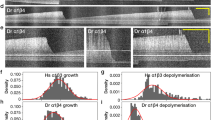
Small-molecule inhibitors of kinesin-5 (refs. 1–3), a protein essential for eukaryotic cell division4, represent alternatives to antimitotic agents that target tubulin5,6. While tubulin is needed for multiple intracellular processes, the known functions of kinesin-5 are limited to dividing cells, making it likely that kinesin-5 inhibitors would have fewer side effects than do tubulin-targeting drugs. Kinesin-5 inhibitors, such as monastrol1, act through poorly understood allosteric mechanisms, not competing with ATP binding7,8. Moreover, the microscopic mechanism of full-length kinesin-5 motility is not known. Here we characterize the motile properties and allosteric inhibition of Eg5, a vertebrate kinesin-5, using a GFP fusion protein in single-molecule fluorescence assays9. We find that Eg5 is a processive kinesin whose motility includes, in addition to ATP-dependent directional motion, a diffusive component not requiring ATP hydrolysis. Monastrol suppresses the directional processive motility of microtubule-bound Eg5. These data on Eg5's allosteric inhibition will impact these inhibitors' use as probes and development as chemotherapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mayer, T.U. et al. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286, 971–974 (1999).
Cox, C.D. et al. Kinesin spindle protein (KSP) inhibitors. Part 1: The discovery of 3,5-diaryl-4,5-dihydropyrazoles as potent and selective inhibitors of the mitotic kinesin KSP. Bioorg. Med. Chem. Lett. 15, 2041–2045 (2005).
DeBonis, S. et al. In vitro screening for inhibitors of the human mitotic kinesin Eg5 with antimitotic and antitumor activities. Mol. Cancer Ther. 3, 1079–1090 (2004).
Sharp, D.J., Rogers, G.C. & Scholey, J.M. Microtubule motors in mitosis. Nature 407, 41–47 (2000).
Jordan, M.A. & Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 4, 253–265 (2004).
Bergnes, G., Brejc, K. & Belmont, L. Mitotic kinesins: prospects for antimitotic drug discovery. Curr. Top. Med. Chem. 5, 127–145 (2005).
Maliga, Z., Kapoor, T.M. & Mitchison, T.J. Evidence that monastrol is an allosteric inhibitor of the mitotic kinesin Eg5. Chem. Biol. 9, 989–996 (2002).
Yan, Y. et al. Inhibition of a mitotic motor protein: where, how, and conformational consequences. J. Mol. Biol. 335, 547–554 (2004).
Peterman, E.J., Sosa, H. & Moerner, W.E. Single-molecule fluorescence spectroscopy and microscopy of biomolecular motors. Annu. Rev. Phys. Chem. 55, 79–96 (2004).
Sawin, K.E., LeGuellec, K., Philippe, M. & Mitchison, T.J. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature 359, 540–543 (1992).
Kashina, A.S. et al. A bipolar kinesin. Nature 379, 270–272 (1996).
Kapitein, L.C. et al. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature 435, 114–118 (2005).
Desai, A., Murray, A., Mitchison, T.J. & Walczak, C.E. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61, 385–412 (1999).
Kwok, B.H., Yang, J.G. & Kapoor, T.M. The rate of bipolar spindle assembly depends on the microtubule-gliding velocity of the mitotic kinesin Eg5. Curr. Biol. 14, 1783–1788 (2004).
Kapoor, T.M. & Mitchison, T.J. Eg5 is static in bipolar spindles relative to tubulin: evidence for a static spindle matrix. J. Cell Biol. 154, 1125–1133 (2001).
Sharp, D.J. et al. The bipolar kinesin, KLP61F, cross-links microtubules within interpolar microtubule bundles of Drosophila embryonic mitotic spindles. J. Cell Biol. 144, 125–138 (1999).
Dickson, R.M., Cubitt, A.B., Tsien, R.Y. & Moerner, W.E. On/off blinking and switching behaviour of single molecules of green fluorescent protein. Nature 388, 355–358 (1997).
Peterman, E.J., Brasselet, S. & Moerner, W.E. The fluorescence dynamics of single molecules of green fluorescent protein. J. Phys. Chem. A 103, 10553–10560 (1999).
Qian, H., Sheetz, M.P. & Elson, E.L. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys. J. 60, 910–921 (1991).
Saxton, M.J. Single-particle tracking: the distribution of diffusion coefficients. Biophys. J. 72, 1744–1753 (1997).
Svoboda, K., Mitra, P.P. & Block, S.M. Fluctuation analysis of motor protein movement and single enzyme kinetics. Proc. Natl. Acad. Sci. USA 91, 11782–11786 (1994).
Okada, Y. & Hirokawa, N. Mechanism of the single-headed processivity: diffusional anchoring between the K-loop of kinesin and the C terminus of tubulin. Proc. Natl. Acad. Sci. USA 97, 640–645 (2000).
Okada, Y. & Hirokawa, N. A processive single-headed motor: kinesin superfamily protein KIF1A. Science 283, 1152–1157 (1999).
Helenius, J., Brouhard, G., Kalaidzidis, Y., Diez, S. & Howard, J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature 441, 115–119 (2006).
Cochran, J.C., Gatial, J.E., III, Kapoor, T.M. & Gilbert, S.P. Monastrol inhibition of the mitotic kinesin Eg5. J. Biol. Chem. 280, 12658–12667 (2005).
Krzysiak, T.C. et al. A structural model for monastrol inhibition of dimeric kinesin Eg5. EMBO J. 25, 2263–2273 (2006).
Turner, J. et al. Crystal structure of the mitotic spindle kinesin Eg5 reveals a novel conformation of the neck-linker. J. Biol. Chem. 276, 25496–25502 (2001).
Crevel, I.M., Alonso, M.C. & Cross, R.A. Monastrol stabilises an attached low-friction mode of Eg5. Curr. Biol. 14, R411–R412 (2004).
Acknowledgements
We thank G. Woehlke and U. Peters for Nkin-GFP and DHP2, respectively. Support was provided in part by a Research Grant from the Human Frontier Science Program (C.F.S. and T.M.K.). C.F.S. acknowledges the Foundation for Fundamental Research on Matter and the DFG Center Molecular Physiology of the Brain. L.C.K. and E.J.G.P. are grateful for a VIDI fellowship (E.J.G.P.) from the Research Council for Earth and Life Sciences (ALW), with financial aid from the Netherlands Organization for Scientific Research (NWO). T.M.K., B.H.K., J.H.K. are grateful to the US National Institutes of Health/National Institute of General Medical Sciences (GM65933) for support. B.H.K. is a Merck postdoctoral fellow.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Purification of TEV protease-cleaved Eg5-GFP fusion protein. (PDF 308 kb)
Supplementary Fig. 2
Eg5-GFP can functionally replace endogenous Eg5 motor. (PDF 176 kb)
Supplementary Fig. 3
Position traces of microtubule-bound Eg5-GFP over time. (PDF 210 kb)
Supplementary Fig. 4
Chemical structure of DHP2, 2. (PDF 160 kb)
Rights and permissions
About this article
Cite this article
Kwok, B., Kapitein, L., Kim, J. et al. Allosteric inhibition of kinesin-5 modulates its processive directional motility. Nat Chem Biol 2, 480–485 (2006). https://doi.org/10.1038/nchembio812
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio812
This article is cited by
-
Small molecule allosteric uncoupling of microtubule depolymerase activity from motility in human Kinesin-5 during mitotic spindle assembly
Scientific Reports (2019)
-
Embedding dual function into molecular motors through collective motion
Scientific Reports (2017)
-
Prime movers: the mechanochemistry of mitotic kinesins
Nature Reviews Molecular Cell Biology (2014)
-
Moving into the cell: single-molecule studies of molecular motors in complex environments
Nature Reviews Molecular Cell Biology (2011)
-
Directionality of individual kinesin-5 Cin8 motors is modulated by loop 8, ionic strength and microtubule geometry
The EMBO Journal (2011)