Abstract
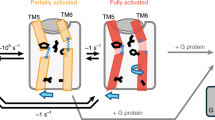
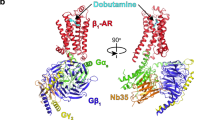
G protein–coupled receptors (GPCRs) regulate a wide variety of physiological functions in response to structurally diverse ligands ranging from cations and small organic molecules to peptides and glycoproteins. For many GPCRs, structurally related ligands can have diverse efficacy profiles. To investigate the process of ligand binding and activation, we used fluorescence spectroscopy to study the ability of ligands having different efficacies to induce a specific conformational change in the human β2-adrenoceptor (β2-AR). The 'ionic lock' is a molecular switch found in rhodopsin-family GPCRs that has been proposed to link the cytoplasmic ends of transmembrane domains 3 and 6 in the inactive state1,2,3. We found that most partial agonists were as effective as full agonists in disrupting the ionic lock. Our results show that disruption of this important molecular switch is necessary, but not sufficient, for full activation of the β2-AR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Ballesteros, J.A. et al. Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 276, 29171–29177 (2001).
Greasley, P.J., Fanelli, F., Rossier, O., Abuin, L. & Cotecchia, S. Mutagenesis and modelling of the alpha(1b)-adrenergic receptor highlight the role of the helix 3/helix 6 interface in receptor activation. Mol. Pharmacol. 61, 1025–1032 (2002).
Shapiro, D.A., Kristiansen, K., Weiner, D.M., Kroeze, W.K. & Roth, B.L. Evidence for a model of agonist-induced activation of 5-hydroxytryptamine 2A serotonin receptors that involves the disruption of a strong ionic interaction between helices 3 and 6. J. Biol. Chem. 277, 11441–11449 (2002).
Farrens, D.L., Altenbach, C., Yang, K., Hubbell, W.L. & Khorana, H.G. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science 274, 768–770 (1996).
Ghanouni, P. et al. Functionally different agonists induce distinct conformations in the G protein coupling domain of the beta 2 adrenergic receptor. J. Biol. Chem. 276, 24433–24436 (2001).
Swaminath, G. et al. Sequential binding of agonists to the beta2 adrenoceptor. Kinetic evidence for intermediate conformational states. J. Biol. Chem. 279, 686–691 (2004).
Swaminath, G. et al. Probing the beta2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J. Biol. Chem. 280, 22165–22171 (2005).
Mansoor, S.E., McHaourab, H.S. & Farrens, D.L. Mapping proximity within proteins using fluorescence spectroscopy. A study of T4 lysozyme showing that tryptophan residues quench bimane fluorescence. Biochemistry 41, 2475–2484 (2002).
Green, S.A., Cole, G., Jacinto, M., Innis, M. & Liggett, S.B. A polymorphism of the human beta 2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J. Biol. Chem. 268, 23116–23121 (1993).
Shi, L. et al. Beta2 adrenergic receptor activation. Modulation of the proline kink in transmembrane 6 by a rotamer toggle switch. J. Biol. Chem. 277, 40989–40996 (2002).
Ruprecht, J.J., Mielke, T., Vogel, R., Villa, C. & Schertler, G.F. Electron crystallography reveals the structure of metarhodopsin I. EMBO J. 23, 3609–3620 (2004).
Hoffmann, C. et al. A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat. Methods 2, 171–176 (2005).
Vilardaga, J.P., Steinmeyer, R., Harms, G.S. & Lohse, M.J. Molecular basis of inverse agonism in a G protein-coupled receptor. Nat. Chem. Biol. 1, 25–28 (2005).
Jongejan, A. et al. Linking agonist binding to histamine H1 receptor activation. Nat. Chem. Biol. 1, 98–103 (2005).
Parnot, C., Miserey-Lenkei, S., Bardin, S., Corvol, P. & Clauser, E. Lessons from constitutively active mutants of G protein-coupled receptors. Trends Endocrinol. Metab. 13, 336–343 (2002).
Gether, U. et al. Agonists induce conformational changes in transmembrane domains III and VI of the beta2 adrenoceptor. EMBO J. 16, 6737–6747 (1997).
Kobilka, B.K. Amino and carboxyl terminal modifications to facilitate the production and purification of a G protein-coupled receptor. Anal. Biochem. 231, 269–271 (1995).
Ballesteros, J. & Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428 (1995).
Li, J., Edwards, P.C., Burghammer, M., Villa, C. & Schertler, G.F. Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol. 343, 1409–1438 (2004).
Wang, J., Wolf, R.M., Caldwell, J.W., Kollman, P.A. & Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Merritt, E.A. & Bacon, D.J. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277, 505–524 (1997).
Acknowledgements
This work was supported by grants from the US National Institutes of Health (Grant 5 RO1 NS28471 to B.K. and Grant R01 DA14896 to D.F.) and the Mather's Charitable Foundation (to B.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Site-specific labeling of Cys271 in the β2-AR. (PDF 658 kb)
Supplementary Fig. 2
Isoproterenol binding to bimane-labeled β2-AR-Δ5-Cys271+Trp135. (PDF 531 kb)
Supplementary Table 1
Equilibrium dissociation constants for ligand binding to the β2-AR-Δ5-Cys271 and β2-AR-Δ5-Cys271+Trp135 receptors. (PDF 99 kb)
Rights and permissions
About this article
Cite this article
Yao, X., Parnot, C., Deupi, X. et al. Coupling ligand structure to specific conformational switches in the β2-adrenoceptor. Nat Chem Biol 2, 417–422 (2006). https://doi.org/10.1038/nchembio801
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio801
This article is cited by
-
Site-Directed Fluorescence Labeling (SDFL): TrIQ Methods Provide Insights Using the Fluorescent Probe Bimane
Applied Magnetic Resonance (2024)
-
The dynamics of agonist-β2-adrenergic receptor activation induced by binding of GDP-bound Gs protein
Nature Chemistry (2023)
-
Novel interaction between neurotrophic factor-α1/carboxypeptidase E and serotonin receptor, 5-HTR1E, protects human neurons against oxidative/neuroexcitotoxic stress via β-arrestin/ERK signaling
Cellular and Molecular Life Sciences (2022)
-
DeSiphering receptor core-induced and ligand-dependent conformational changes in arrestin via genetic encoded trimethylsilyl 1H-NMR probe
Nature Communications (2020)
-
GPCR drug discovery: integrating solution NMR data with crystal and cryo-EM structures
Nature Reviews Drug Discovery (2019)