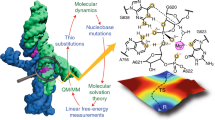
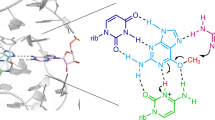
Abstract
RNA enzymes (ribozymes) have remarkably diverse biological roles despite having limited chemical diversity. Protein enzymes enhance their reactivity through recruitment of cofactors; likewise, the naturally occurring glmS ribozyme uses the glucosamine-6-phosphate (GlcN6P) organic cofactor for phosphodiester bond cleavage. Prior structural and biochemical studies have implicated GlcN6P as the general acid. Here we describe new catalytic roles of GlcN6P through experiments and calculations. Large stereospecific normal thio effects and a lack of metal-ion rescue in the holoribozyme indicate that nucleobases and the cofactor play direct chemical roles and align the active site for self-cleavage. Large stereospecific inverse thio effects in the aporibozyme suggest that the GlcN6P cofactor disrupts an inhibitory interaction of the nucleophile. Strong metal-ion rescue in the aporibozyme reveals that this cofactor also provides electrostatic stabilization. Ribozyme organic cofactors thus perform myriad catalytic roles, thereby allowing RNA to compensate for its limited functional diversity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Li, Y. & Breaker, R.R. Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2′-hydroxyl group. J. Am. Chem. Soc. 121, 5364–5372 (1999).
Soukup, G.A. & Breaker, R.R. Relationship between internucleotide linkage geometry and the stability of RNA. RNA 5, 1308–1325 (1999).
Emilsson, G.M., Nakamura, S., Roth, A. & Breaker, R.R. Ribozyme speed limits. RNA 9, 907–918 (2003).
Fersht, A. Structure and Mechanism in Protein Science (W.H. Freeman, 1999).
Cech, T.R., Zaug, A.J. & Grabowski, P.J. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell 27, 487–496 (1981).
Viladoms, J., Scott, L.G. & Fedor, M.J. An active-site guanine participates in glmS ribozyme catalysis in its protonated state. J. Am. Chem. Soc. 133, 18388–18396 (2011).
Soukup, J.K. The structural and functional uniqueness of the glmS ribozyme. Prog. Mol. Biol. Transl. Sci. 120, 173–193 (2013).
Zhang, S. et al. Role of the active site guanine in the glmS ribozyme self-cleavage mechanism: quantum mechanical/molecular mechanical free energy simulations. J. Am. Chem. Soc. 137, 784–798 (2015).
Klein, D.J. & Ferré-D'Amaré, A.R. Structural basis of glmS ribozyme activation by glucosamine-6-phosphate. Science 313, 1752–1756 (2006).
Cochrane, J.C., Lipchock, S.V. & Strobel, S.A. Structural investigation of the glmS ribozyme bound to its catalytic cofactor. Chem. Biol. 14, 97–105 (2007).
Viladoms, J. & Fedor, M.J. The glmS ribozyme cofactor is a general acid-base catalyst. J. Am. Chem. Soc. 134, 19043–19049 (2012).
Klein, D.J., Wilkinson, S.R., Been, M.D. & Ferré-D'Amaré, A.R. Requirement of helix P2.2 and nucleotide G1 for positioning the cleavage site and cofactor of the glmS ribozyme. J. Mol. Biol. 373, 178–189 (2007).
Uhlenbeck, O.C. Keeping RNA happy. RNA 1, 4–6 (1995).
Chadalavada, D.M., Senchak, S.E. & Bevilacqua, P.C. The folding pathway of the genomic hepatitis delta virus ribozyme is dominated by slow folding of the pseudoknots. J. Mol. Biol. 317, 559–575 (2002).
Brown, T.S., Chadalavada, D.M. & Bevilacqua, P.C. Design of a highly reactive HDV ribozyme sequence uncovers facilitation of RNA folding by alternative pairings and physiological ionic strength. J. Mol. Biol. 341, 695–712 (2004).
Roth, A., Nahvi, A., Lee, M., Jona, I. & Breaker, R.R. Characteristics of the glmS ribozyme suggest only structural roles for divalent metal ions. RNA 12, 607–619 (2006).
Frederiksen, J.K. & Piccirilli, J.A. Identification of catalytic metal ion ligands in ribozymes. Methods 49, 148–166 (2009).
Klawuhn, K., Jansen, J.A., Souchek, J., Soukup, G.A. & Soukup, J.K. Analysis of metal ion dependence in glmS ribozyme self-cleavage and coenzyme binding. ChemBioChem 11, 2567–2571 (2010).
Brooks, K.M. & Hampel, K.J. Rapid steps in the glmS ribozyme catalytic pathway: cation and ligand requirements. Biochemistry 50, 2424–2433 (2011).
Murray, J.B., Seyhan, A.A., Walter, N.G., Burke, J.M. & Scott, W.G. The hammerhead, hairpin and VS ribozymes are catalytically proficient in monovalent cations alone. Chem. Biol. 5, 587–595 (1998).
Perrotta, A.T. & Been, M.D. HDV ribozyme activity in monovalent cations. Biochemistry 45, 11357–11365 (2006).
Frederiksen, J.K., Li, N.-S., Das, R., Herschlag, D. & Piccirilli, J.A. Metal-ion rescue revisited: biochemical detection of site-bound metal ions important for RNA folding. RNA 18, 1123–1141 (2012).
Hampel, K.J. & Tinsley, M.M. Evidence for preorganization of the glmS ribozyme ligand binding pocket. Biochemistry 45, 7861–7871 (2006).
Thaplyal, P., Ganguly, A., Hammes-Schiffer, S. & Bevilacqua, P.C. Inverse thio effects in the hepatitis delta virus ribozyme reveal that the reaction pathway is controlled by metal ion charge density. Biochemistry 54, 2160–2175 (2015).
DeRose, V.J. Metal ion binding to catalytic RNA molecules. Curr. Opin. Struct. Biol. 13, 317–324 (2003).
Johnson-Buck, A.E., McDowell, S.E. & Walter, N.G. Metal ions: supporting actors in the playbook of small ribozymes. Met. Ions Life Sci. 9, 175–196 (2011).
Bevilacqua, P.C. & Yajima, R. Nucleobase catalysis in ribozyme mechanism. Curr. Opin. Chem. Biol. 10, 455–464 (2006).
Cochrane, J.C., Lipchock, S.V., Smith, K.D. & Strobel, S.A. Structural and chemical basis for glucosamine 6-phosphate binding and activation of the glmS ribozyme. Biochemistry 48, 3239–3246 (2009).
Brooks, K.M. & Hampel, K.J. A rate-limiting conformational step in the catalytic pathway of the glmS ribozyme. Biochemistry 48, 5669–5678 (2009).
Scott, E.C. & Uhlenbeck, O.C. A re-investigation of the thio effect at the hammerhead cleavage site. Nucleic Acids Res. 27, 479–484 (1999).
Yoshida, A., Sun, S. & Piccirilli, J.A. A new metal ion interaction in the Tetrahymena ribozyme reaction revealed by double sulfur substitution. Nat. Struct. Biol. 6, 318–321 (1999).
Ward, W.L. & Derose, V.J. Ground-state coordination of a catalytic metal to the scissile phosphate of a tertiary-stabilized Hammerhead ribozyme. RNA 18, 16–23 (2012).
Jencks, W.P. Catalysis in Chemistry and Enzymology (Dover, 1969).
Zhang, S. et al. Assessing the potential effects of active site Mg2+ ions in the glmS ribozyme–cofactor complex. J. Phys. Chem. Lett. 7, 3984–3988 (2016).
Lau, M.W.L. & Ferré-D'Amaré, A.R. An in vitro evolved glmS ribozyme has the wild-type fold but loses coenzyme dependence. Nat. Chem. Biol. 9, 805–810 (2013).
Chinnapen, D.J.-F. & Sen, D. A deoxyribozyme that harnesses light to repair thymine dimers in DNA. Proc. Natl. Acad. Sci. USA 101, 65–69 (2004).
Cernak, P. & Sen, D. A thiamin-utilizing ribozyme decarboxylates a pyruvate-like substrate. Nat. Chem. 5, 971–977 (2013).
Poon, L.C.H. et al. Guanine-rich RNAs and DNAs that bind heme robustly catalyze oxygen transfer reactions. J. Am. Chem. Soc. 133, 1877–1884 (2011).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Acerbo, A.S., Cook, M.J. & Gillilan, R.E. Upgrade of MacCHESS facility for X-ray scattering of biological macromolecules in solution. J. Synchrotron Radiat. 22, 180–186 (2015).
Skou, S., Gillilan, R.E. & Ando, N. Synchrotron-based small-angle X-ray scattering of proteins in solution. Nat. Protoc. 9, 1727–1739 (2014).
Nielsen, S.S. et al. BioXTAS RAW, a software program for high-throughput automated small-angle X-ray scattering data reduction and preliminary analysis. J. Appl. Crystallogr. 42, 959–964 (2009).
Petoukhov, M.V. et al. New developments in the ATSAS program package for small-angle scattering data analysis. J. Appl. Crystallogr. 45, 342–350 (2012).
DeLano, W.L. The PyMOL Molecular Graphics System (DeLano Scientific, 2002).
Rambo, R.P. & Tainer, J.A. Improving small-angle X-ray scattering data for structural analyses of the RNA world. RNA 16, 638–646 (2010).
Lipfert, J., Herschlag, D. & Doniach, S. Riboswitch conformations revealed by small-angle X-ray scattering. in Riboswitches: Methods and Protocols. (ed. Serganov, A.) 141–159 (Humana, 2009).
Case, D.A. et al. AMBER 14 (University of California, San Francisco, 2014).
Yildirim, I., Stern, H.A., Kennedy, S.D., Tubbs, J.D. & Turner, D.H. Reparameterization of RNA χ torsion parameters for the AMBER force field and comparison to NMR Spectra for cytidine and uridine. J. Chem. Theory Comput. 6, 1520–1531 (2010).
Allnér, O., Nilsson, L. & Villa, A. Magnesium ion–water coordination and exchange in biomolecular simulations. J. Chem. Theory Comput. 8, 1493–1502 (2012).
E., W., Ren, W. & Vanden-Eijnden, E. Finite temperature string method for the study of rare events. J. Phys. Chem. B 109, 6688–6693 (2005).
Torrie, G.M. & Valleau, J.P. Nonphysical sampling distributions in Monte Carlo free-energy estimation: umbrella sampling. J. Comput. Phys. 23, 187–199 (1977).
Rosta, E., Nowotny, M., Yang, W. & Hummer, G. Catalytic mechanism of RNA backbone cleavage by ribonuclease H from quantum mechanics/molecular mechanics simulations. J. Am. Chem. Soc. 133, 8934–8941 (2011).
Ganguly, A., Thaplyal, P., Rosta, E., Bevilacqua, P.C. & Hammes-Schiffer, S. Quantum mechanical/molecular mechanical free energy simulations of the self-cleavage reaction in the hepatitis delta virus ribozyme. J. Am. Chem. Soc. 136, 1483–1496 (2014).
Brooks, B.R. et al. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 4, 187–217 (1983).
Kumar, S., Rosenberg, J.M., Bouzida, D., Swendsen, R.H. & Kollman, P.A. The weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J. Comput. Chem. 13, 1011–1021 (1992).
Shao, Y. et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 8, 3172–3191 (2006).
Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 37, 785–789 (1988).
Vosko, S.H., Wilk, L. & Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can. J. Phys. 58, 1200–1211 (1980).
Stephens, P.J., Devlin, F.J., Chabalowski, C.F. & Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 (1994).
Acknowledgements
We thank M. Been (Duke University Medical Center) for the generous gift of the plasmid containing the glmS ribozyme sequence, and we thank R. Gillilan and L. Pollack for help with SAXS experiments. We also thank E. Frankel and K. Leamy for assistance with fast hand-mixing reaction time points. Finally, we thank E. Frankel, R. Poudyal, L. Ritchey, and P. Thaplyal for helpful comments on revising the manuscript. This work was supported by US National Institutes of Health grant GM056207 (S.Z., D.R.S., and S.H.-S.) and US National Science Foundation grant CHE-1213667 (J.L.B. and P.C.B.). D.R.S. is supported as a member of the National Institutes of Health Chemistry-Biology Interface (training grant NRSA 1-T-32-GM070421). This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the National Science Foundation. This work was based on research conducted at the Cornell High Energy Synchrotron Source (CHESS), which is supported by the National Science Foundation and the National Institutes of Health/National Institute of General Medical Sciences under NSF award DMR-0936384, in the Macromolecular Diffraction at CHESS (MacCHESS) facility, which is supported by award GM-103485 from the National Institutes of Health, through the National Institute of General Medical Sciences. We also thank the Penn State Proteomics and Mass Spectrometry Core Facility (University Park, PA) and the Penn State Genomics Core Facility (University Park, PA).
Author information
Authors and Affiliations
Contributions
J.L.B. and P.C.B. designed experiments. J.L.B. performed the biochemistry experiments. J.L.B., N.H.Y., and P.C.B. collected and analyzed SAXS data. S.Z., D.R.S., and S.H.-S. designed calculations. S.Z. and D.R.S. performed the calculations. All authors wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–6 and Supplementary Figures 1–7 (PDF 1828 kb)
Rights and permissions
About this article
Cite this article
Bingaman, J., Zhang, S., Stevens, D. et al. The GlcN6P cofactor plays multiple catalytic roles in the glmS ribozyme. Nat Chem Biol 13, 439–445 (2017). https://doi.org/10.1038/nchembio.2300
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2300