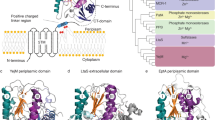
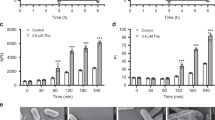
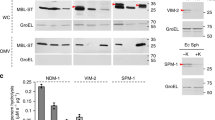
Abstract
Carbapenems, 'last-resort' β-lactam antibiotics, are inactivated by zinc-dependent metallo-β-lactamases (MBLs). The host innate immune response withholds nutrient metal ions from microbial pathogens by releasing metal-chelating proteins such as calprotectin. We show that metal sequestration is detrimental for the accumulation of MBLs in the bacterial periplasm, because those enzymes are readily degraded in their nonmetallated form. However, the New Delhi metallo-β-lactamase (NDM-1) can persist under conditions of metal depletion. NDM-1 is a lipidated protein that anchors to the outer membrane of Gram-negative bacteria. Membrane anchoring contributes to the unusual stability of NDM-1 and favors secretion of this enzyme in outer-membrane vesicles (OMVs). OMVs containing NDM-1 can protect nearby populations of bacteria from otherwise lethal antibiotic levels, and OMVs from clinical pathogens expressing NDM-1 can carry this MBL and the blaNDM gene. We show that protein export into OMVs can be targeted, providing possibilities of new antibacterial therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hede, K. Antibiotic resistance: an infectious arms race. Nature 509, S2–S3 (2014).
Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States 2013 (CDC, Atlanta, Georgia, USA, 2013).
Patel, G. & Bonomo, R.A. “Stormy waters ahead”: global emergence of carbapenemases. Front. Microbiol. 4, 48 (2013).
Crowder, M.W., Spencer, J. & Vila, A.J. Metallo-β-lactamases: novel weaponry for antibiotic resistance in bacteria. Acc. Chem. Res. 39, 721–728 (2006).
Walsh, T.R., Toleman, M.A., Poirel, L. & Nordmann, P. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18, 306–325 (2005).
Dortet, L., Poirel, L. & Nordmann, P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. BioMed Res. Int. 2014, 249856 (2014).
Walsh, T.R., Weeks, J., Livermore, D.M. & Toleman, M.A. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect. Dis. 11, 355–362 (2011).
Zhang, C. et al. Higher isolation of NDM-1 producing Acinetobacter baumannii from the sewage of the hospitals in Beijing. PLoS One 8, e64857 (2014).
Luo, Y. et al. Proliferation of nultidrug-resistant New Delhi metallo-β-lactamase genes in municipal wastewater treatment plants in northern China. Environ. Sci. Technol. Lett. 1, 26–30 (2014).
Poirel, L., Dortet, L., Bernabeu, S. & Nordmann, P. Genetic features of blaNDM-1-positive Enterobacteriaceae. Antimicrob. Agents Chemother. 55, 5403–5407 (2011).
González, J.M. et al. Metallo-β-lactamases withstand low Zn(II) conditions by tuning metal-ligand interactions. Nat. Chem. Biol. 8, 698–700 (2012).
Morán-Barrio, J., Limansky, A.S. & Viale, A.M. Secretion of GOB metallo-β-lactamase in Escherichia coli depends strictly on the cooperation between the cytoplasmic DnaK chaperone system and the Sec machinery: completion of folding and Zn(II) ion acquisition occur in the bacterial periplasm. Antimicrob. Agents Chemother. 53, 2908–2917 (2009).
Cerasi, M., Ammendola, S. & Battistoni, A. Competition for zinc binding in the host-pathogen interaction. Front. Cell. Infect. Microbiol. 3, 108 (2013).
Hood, M.I. & Skaar, E.P. Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 10, 525–537 (2012).
Brophy, M.B., Hayden, J.A. & Nolan, E.M. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J. Am. Chem. Soc. 134, 18089–18100 (2012).
Corbin, B.D. et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319, 962–965 (2008).
Meini, M.R., Tomatis, P.E., Weinreich, D.M. & Vila, A.J. Quantitative description of a protein fitness landscape based on molecular features. Mol. Biol. Evol. 32, 1774–1787 (2015).
Schwechheimer, C. & Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619 (2015).
González, L.J., Moreno, D.M., Bonomo, R.A. & Vila, A.J. Host-specific enzyme-substrate interactions in SPM-1 metallo-β-lactamase are modulated by second sphere residues. PLoS Pathog. 10, e1003817 (2014).
King, D. & Strynadka, N. Crystal structure of New Delhi metallo-β-lactamase reveals molecular basis for antibiotic resistance. Protein Sci. 20, 1484–1491 (2011).
Kovacs-Simon, A., Titball, R.W. & Michell, S.L. Lipoproteins of bacterial pathogens. Infect. Immun. 79, 548–561 (2011).
Rokney, A. et al. E. coli transports aggregated proteins to the poles by a specific and energy-dependent process. J. Mol. Biol. 392, 589–601 (2009).
Hussain, M., Ichihara, S. & Mizushima, S. Accumulation of glyceride-containing precursor of the outer membrane lipoprotein in the cytoplasmic membrane of Escherichia coli treated with globomycin. J. Biol. Chem. 255, 3707–3712 (1980).
Randall, L.B., Dobos, K., Papp-Wallace, K.M., Bonomo, R.A. & Schweizer, H.P. Membrane-bound PenA β-lactamase of Burkholderia pseudomallei. Antimicrob. Agents Chemother. 60, 1509–1514 (2015).
Bootsma, H.J., van Dijk, H., Verhoef, J., Fleer, A. & Mooi, F.R. Molecular characterization of the BRO β-lactamase of Moraxella (Branhamella) catarrhalis. Antimicrob. Agents Chemother. 40, 966–972 (1996).
Schaar, V., Nordström, T., Mörgelin, M. & Riesbeck, K. Moraxella catarrhalis outer membrane vesicles carry β-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob. Agents Chemother. 55, 3845–3853 (2011).
Bomberger, J.M. et al. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5, e1000382 (2009).
Devos, S. et al. The effect of imipenem and diffusible signaling factors on the secretion of outer membrane vesicles and associated Ax21 proteins in Stenotrophomonas maltophilia. Front. Microbiol. 6, 298 (2015).
Schaar, V., Uddbäck, I., Nordström, T. & Riesbeck, K. Group A streptococci are protected from amoxicillin-mediated killing by vesicles containing β-lactamase derived from Haemophilus influenzae. J. Antimicrob. Chemother. 69, 117–120 (2014).
Rumbo, C. et al. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 55, 3084–3090 (2011).
Tottey, S. et al. Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature 455, 1138–1142 (2008).
Hu, Z., Gunasekera, T.S., Spadafora, L., Bennett, B. & Crowder, M.W. Metal content of metallo-β-lactamase L1 is determined by the bioavailability of metal ions. Biochemistry 47, 7947–7953 (2008).
Kim, Y. et al. Structure of apo- and monometalated forms of NDM-1--a highly potent carbapenem-hydrolyzing metallo-β-lactamase. PLoS One 6, e24621 (2011).
González, J.M., Buschiazzo, A. & Vila, A.J. Evidence of adaptability in metal coordination geometry and active-site loop conformation among B1 metallo-β-lactamases. Biochemistry 49, 7930–7938 (2010).
Borra, P.S. et al. Crystal structures of Pseudomonas aeruginosa GIM-1: active-site plasticity in metallo-β-lactamases. Antimicrob. Agents Chemother. 57, 848–854 (2013).
Hernandez Valladares, M. et al. Zn(II) dependence of the Aeromonas hydrophila AE036 metallo-β-lactamase activity and stability. Biochemistry 36, 11534–11541 (1997).
Selevsek, N. et al. Zinc ion-induced domain organization in metallo-β-lactamases: a flexible “zinc arm” for rapid metal ion transfer? J. Biol. Chem. 284, 16419–16431 (2009).
Periyannan, G., Shaw, P.J., Sigdel, T. & Crowder, M.W. In vivo folding of recombinant metallo-β-lactamase L1 requires the presence of Zn(II). Protein Sci. 13, 2236–2243 (2004).
Wommer, S. et al. Substrate-activated zinc binding of metallo-β-lactamases: physiological importance of mononuclear enzymes. J. Biol. Chem. 277, 24142–24147 (2002).
Johne, B. et al. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol. Pathol. 50, 113–123 (1997).
Haley, K.P. et al. The human antimicrobial protein calgranulin C participates in control of Helicobacter pylori growth and regulation of virulence. Infect. Immun. 83, 2944–2956 (2015).
Gläser, R. et al. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 6, 57–64 (2005).
Pederick, V.G. et al. ZnuA and zinc homeostasis in Pseudomonas aeruginosa. Sci. Rep. 5, 13139 (2015).
Kesty, N.C. & Kuehn, M.J. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J. Biol. Chem. 279, 2069–2076 (2004).
Haurat, M.F. et al. Selective sorting of cargo proteins into bacterial membrane vesicles. J. Biol. Chem. 286, 1269–1276 (2011).
Veith, P.D. et al. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J. Proteome Res. 13, 2420–2432 (2014).
Santos, S. et al. Outer membrane vesicles (OMV) production of Neisseria meningitidis serogroup B in batch process. Vaccine 30, 6064–6069 (2012).
Irazoqui, J.E. et al. Distinct pathogenesis and host responses during infection of C. elegans by P. aeruginosa and S. aureus. PLoS Pathog. 6, e1000982 (2010).
Kitagawa, R. et al. Biogenesis of Salmonella enterica serovar typhimurium membrane vesicles provoked by induction of PagC. J. Bacteriol. 192, 5645–5656 (2010).
Murphy, T.A., Simm, A.M., Toleman, M.A., Jones, R.N. & Walsh, T.R. Biochemical characterization of the acquired metallo-β-lactamase SPM-1 from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 47, 582–587 (2003).
Fiorilli, G. et al. Emergence of metallo-β-lactamases in Acinetobacter spp clinical isolates from Argentina. Rev. Esp. Quimioter. 23, 100–102 (2010).
Marchiaro, P. et al. Biochemical characterization of metallo-β-lactamase VIM-11 from a Pseudomonas aeruginosa clinical strain. Antimicrob. Agents Chemother. 52, 2250–2252 (2008).
Ravasi, P., Peiru, S., Gramajo, H. & Menzella, H.G. Design and testing of a synthetic biology framework for genetic engineering of Corynebacterium glutamicum. Microb. Cell Fact. 11, 147 (2012).
Kovach, M.E. et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166, 175–176 (1995).
Choi, K.H., Kumar, A. & Schweizer, H.P. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64, 391–397 (2006).
Schneider, C.A., Rasband, W.S. & Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Chen, X., Brown, T. & Tai, P.C. Identification and characterization of protease-resistant SecA fragments: AecA has two membrane-integral forms. J. Bacteriol. 180, 527–537 (1998).
Aschtgen, M.S., Bernard, C.S., De Bentzmann, S., Lloubès, R. & Cascales, E. SciN is an outer membrane lipoprotein required for type VI secretion in enteroaggregative Escherichia coli. J. Bacteriol. 190, 7523–7531 (2008).
CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard - Ninth Edition. CLSI Document M07-A9 (Clinical Laboratory Standards Institute, Wayne, Pennsylvania, USA, 2012).
Park, M. et al. Isolation and characterization of the outer membrane of Escherichia coli with autodisplayed Z-domains. Biochim. Biophys. Acta 1848, 842–847 (2015).
Acknowledgements
We thank J. Spencer (University of Bristol), H. Menzella (University of Rosario) and R. Rasia (IBR, CONICET-UNR) for providing plasmids pET26-blaNDM-1, pTGR11 and pET28-TEV, respectively, A. Corso (Administración Nacional de Laboratorios e Instituos de Salud) for providing clinical strains, and A. Viale (IBR) for GroEL antibodies. This research was supported by grants from the US National Institutes of Health (NIH; R01AI100560) to A.J.V. and R.A.B., and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) to A.J.V. R.A.B. acknowledges support from NIH under award numbers R01AI072219, R01AI063517 and R01AI100560, and funds and/or facilities provided by the Louis Stokes Cleveland Department of Veterans Affairs Medical Center and the VISN 10 Geriatric Research, Education and Clinical Care Center (VISN 10) of the Department of Veterans Affairs. E.M.N. thanks support from the Kinship Foundation (Searle Scholars Program) and MIT Department of Chemistry.
Author information
Authors and Affiliations
Contributions
L.J.G. and G.B. performed the microbiological, molecular biology and biochemical experiments. T.G.N. purified calprotectin. L.J.G., G.B., T.G.N., E.M.N., R.A.B. and A.J.V. analyzed and discussed data. L.J.G., G.B., R.A.B. and A.J.V. wrote the paper, and all authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–13 and Supplementary Tables 1–5. (PDF 2202 kb)
Rights and permissions
About this article
Cite this article
González, L., Bahr, G., Nakashige, T. et al. Membrane anchoring stabilizes and favors secretion of New Delhi metallo-β-lactamase. Nat Chem Biol 12, 516–522 (2016). https://doi.org/10.1038/nchembio.2083
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2083
This article is cited by
-
Identification and characterization of CIM-1, a carbapenemase that adds to the family of resistance factors against last resort antibiotics
Communications Biology (2024)
-
Role of membrane vesicles in the transmission of vancomycin resistance in Enterococcus faecium
Scientific Reports (2024)
-
A critical role of outer membrane vesicles in antibiotic resistance in carbapenem-resistant Klebsiella pneumoniae
Annals of Clinical Microbiology and Antimicrobials (2023)
-
In-cell protein stability promotes antimicrobial resistance of metallo-β-lactamases
Nature Chemical Biology (2023)
-
Composition and functions of bacterial membrane vesicles
Nature Reviews Microbiology (2023)