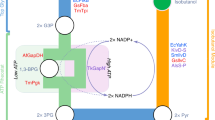
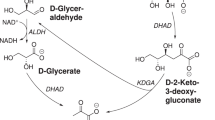
Abstract
Synthetic biochemistry, the cell-free production of biologically based chemicals, is a potentially high-yield, flexible alternative to in vivo metabolic engineering. To limit costs, cell-free systems must be designed to operate continuously with minimal addition of feedstock chemicals. We describe a robust, efficient synthetic glucose breakdown pathway and implement it for the production of bioplastic. The system's performance suggests that synthetic biochemistry has the potential to become a viable industrial alternative.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Zhang, Y.-H.P. ACS Catal. 1, 998–1009 (2011).
Yu, X., Liu, T., Zhu, F. & Khosla, C. Proc. Natl. Acad. Sci. USA 108, 18643–18648 (2011).
Schultheisz, H.L., Szymczyna, B.R., Scott, L.G. & Williamson, J.R. J. Am. Chem. Soc. 133, 297–304 (2011).
Schultheisz, H.L., Szymczyna, B.R., Scott, L.G. & Williamson, J.R. ACS Chem. Biol. 3, 499–511 (2008).
Rollin, J.A., Tam, T.K. & Zhang, Y.-H.P. Green Chem. 15, 1708–1719 (2013).
Jewett, M.C. & Swartz, J.R. Biotechnol. Prog. 20, 102–109 (2004).
Hold, C. & Panke, S. J. R. Soc. Interface 6 (suppl. 4), S507–S521 (2009).
Bujara, M., Schümperli, M., Billerbeck, S., Heinemann, M. & Panke, S. Biotechnol. Bioeng. 106, 376–389 (2010).
Zhang, Y.-H.P. Biotechnol. Bioeng. 105, 663–677 (2010).
Verlinden, R.A., Hill, D.J., Kenward, M.A., Williams, C.D. & Radecka, I. J. Appl. Microbiol. 102, 1437–1449 (2007).
Singh, M., Kumar, P., Ray, S. & Kalia, V.C. Indian J. Microbiol. 55, 235–249 (2015).
Hankermeyer, C.R. & Tjeerdema, R.S. Rev. Environ. Contam. Toxicol. 159, 1–24 (1999).
Satoh, Y., Tajima, K., Tannai, H. & Munekata, M. J. Biosci. Bioeng. 95, 335–341 (2003).
Jossek, R. & Steinbüchel, A. FEMS Microbiol. Lett. 168, 319–324 (1998).
Liu, S.-J. & Steinbüchel, A. Appl. Microbiol. Biotechnol. 53, 545–552 (2000).
Opgenorth, P.H., Korman, T.P. & Bowie, J.U. Nat. Commun. 5, 4113 (2014).
Meile, L., Rohr, L.M., Geissmann, T.A., Herensperger, M. & Teuber, M. J. Bacteriol. 183, 2929–2936 (2001).
Glenn, K. & Smith, K.S. J. Bacteriol. 197, 1157–1163 (2015).
Zhang, S., Yasuo, T., Lenz, R.W. & Goodwin, S. Biomacromolecules 1, 244–251 (2000).
Dien, B.S., Cotta, M.A. & Jeffries, T.W. Appl. Microbiol. Biotechnol. 63, 258–266 (2003).
Gibson, D.G. et al. Science 319, 1215–1220 (2008).
Kitagawa, M. et al. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 12, 291–299 (2005).
Sheu, D.-S., Chen, W.-M., Lai, Y.-W. & Chang, R.-C. J. Bacteriol. 194, 2620–2629 (2012).
Acknowledgements
This work was supported by US DOE grant DE-FC02-02ER63421 and ARPA-E grant DE-AR0000556.
Author information
Authors and Affiliations
Contributions
All authors contributed to the system design, design of experiments and data analysis. P.O. and T.P.K. performed the experiments. All the authors wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors have formed a company, Invizyne Technologies, that will seek to exploit cell-free technologies.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1 and 2, Supplementary Tables 1–3 and Supplementary Note. (PDF 438 kb)
Rights and permissions
About this article
Cite this article
Opgenorth, P., Korman, T. & Bowie, J. A synthetic biochemistry module for production of bio-based chemicals from glucose. Nat Chem Biol 12, 393–395 (2016). https://doi.org/10.1038/nchembio.2062
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2062
This article is cited by
-
Using a synthetic machinery to improve carbon yield with acetylphosphate as the core
Nature Communications (2023)
-
Opportunities and Challenges of in vitro Synthetic Biosystem for Terpenoids Production
Biotechnology and Bioprocess Engineering (2022)
-
An ATP-free in vitro synthetic enzymatic biosystem facilitating one-pot stoichiometric conversion of starch to mannitol
Applied Microbiology and Biotechnology (2021)
-
Engineering the transmission efficiency of the noncyclic glyoxylate pathway for fumarate production in Escherichia coli
Biotechnology for Biofuels (2020)
-
A roadmap towards integrated catalytic systems of the future
Nature Catalysis (2020)