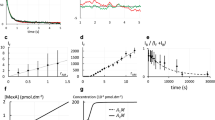
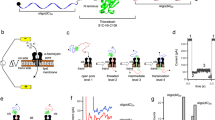
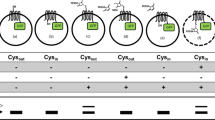
Abstract
Membrane proteins are assembled through balanced interactions among proteins, lipids and water. Studying their folding while maintaining the native lipid environment is necessary but challenging. Here we present methods for analyzing key elements of membrane protein folding including thermodynamic stability, compactness of the unfolded state and folding cooperativity under native conditions. The methods are based on steric trapping, which couples the unfolding of a doubly biotinylated protein to the binding of monovalent streptavidin (mSA). We further advanced this technology for general application by developing versatile biotin probes possessing spectroscopic reporters that are sensitized by mSA binding or protein unfolding. By applying these methods to the Escherichia coli intramembrane protease GlpG, we elucidated a widely unraveled unfolded state, subglobal unfolding of the region encompassing the active site, and a network of cooperative and localized interactions to maintain stability. These findings provide crucial insights into the folding energy landscape of membrane proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Bryngelson, J.D., Onuchic, J.N., Socci, N.D. & Wolynes, P.G. Funnels, pathways, and the energy landscape of protein folding: a synthesis. Proteins 21, 167–195 (1995).
Oliveberg, M. & Wolynes, P.G. The experimental survey of protein-folding energy landscapes. Q. Rev. Biophys. 38, 245–288 (2005).
Dill, K.A. & Chan, H.S. From Levinthal to pathways to funnels. Nat. Struct. Biol. 4, 10–19 (1997).
Chamberlain, A.K., Handel, T.M. & Marqusee, S. Detection of rare partially folded molecules in equilibrium with the native conformation of RNaseH. Nat. Struct. Biol. 3, 782–787 (1996).
Bai, Y., Sosnick, T.R., Mayne, L. & Englander, S.W. Protein folding intermediates: native-state hydrogen exchange. Science 269, 192–197 (1995).
Sekhar, A. & Kay, L.E. NMR paves the way for atomic level descriptions of sparsely populated, transiently formed biomolecular conformers. Proc. Natl. Acad. Sci. USA 110, 12867–12874 (2013).
Park, C. & Marqusee, S. Probing the high energy states in proteins by proteolysis. J. Mol. Biol. 343, 1467–1476 (2004).
Sharon, M. & Robinson, C.V. The role of mass spectrometry in structure elucidation of dynamic protein complexes. Annu. Rev. Biochem. 76, 167–193 (2007).
Bai, Y. & Englander, S.W. Future directions in folding: the multi-state nature of protein structure. Proteins 24, 145–151 (1996).
Cui, Q. & Karplus, M. Allostery and cooperativity revisited. Protein Sci. 17, 1295–1307 (2008).
Nussinov, R. & Tsai, C.J. Allostery in disease and in drug discovery. Cell 153, 293–305 (2013).
le Coutre, J., Kaback, H.R., Patel, C.K., Heginbotham, L. & Miller, C. Fourier transform infrared spectroscopy reveals a rigid alpha-helical assembly for the tetrameric Streptomyces lividans K+ channel. Proc. Natl. Acad. Sci. USA 95, 6114–6117 (1998).
Joh, N.H. et al. Modest stabilization by most hydrogen-bonded side-chain interactions in membrane proteins. Nature 453, 1266–1270 (2008).
Blois, T.M., Hong, H., Kim, T.H. & Bowie, J.U. Protein unfolding with a steric trap. J. Am. Chem. Soc. 131, 13914–13915 (2009).
Chang, Y.C. & Bowie, J.U. Measuring membrane protein stability under native conditions. Proc. Natl. Acad. Sci. USA 111, 219–224 (2014).
Hong, H., Blois, T.M., Cao, Z. & Bowie, J.U. Method to measure strong protein-protein interactions in lipid bilayers using a steric trap. Proc. Natl. Acad. Sci. USA 107, 19802–19807 (2010).
Hong, H. & Bowie, J.U. Dramatic destabilization of transmembrane helix interactions by features of natural membrane environments. J. Am. Chem. Soc. 133, 11389–11398 (2011).
Jefferson, R.E., Blois, T.M. & Bowie, J.U. Membrane proteins can have high kinetic stability. J. Am. Chem. Soc. 135, 15183–15190 (2013).
Lee, J.R., Urban, S., Garvey, C.F. & Freeman, M. Regulated intracellular ligand transport and proteolysis control EGF signal activation in Drosophila. Cell 107, 161–171 (2001).
McQuibban, G.A., Saurya, S. & Freeman, M. Mitochondrial membrane remodelling regulated by a conserved rhomboid protease. Nature 423, 537–541 (2003).
Stevenson, L.G. et al. Rhomboid protease AarA mediates quorum-sensing in Providencia stuartii by activating TatA of the twin-arginine translocase. Proc. Natl. Acad. Sci. USA 104, 1003–1008 (2007).
Urban, S., Lee, J.R. & Freeman, M. Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell 107, 173–182 (2001).
Baker, R.P. & Urban, S. Architectural and thermodynamic principles underlying intramembrane protease function. Nat. Chem. Biol. 8, 759–768 (2012).
Paslawski, W. et al. Cooperative folding of a polytopic α-helical membrane protein involves a compact N-terminal nucleus and nonnative loops. Proc. Natl. Acad. Sci. USA 112, 7978–7983 (2015).
Min, D., Jefferson, R.E., Bowie, J.U. & Yoon, T.Y. Mapping the energy landscape for second-stage folding of a single membrane protein. Nat. Chem. Biol. 11, 981–987 (2015).
Vosyka, O. et al. Activity-based probes for rhomboid proteases discovered in a mass spectrometry-based assay. Proc. Natl. Acad. Sci. USA 110, 2472–2477 (2013).
Zoll, S. et al. Substrate binding and specificity of rhomboid intramembrane protease revealed by substrate-peptide complex structures. EMBO J. 33, 2408–2421 (2014).
Sherratt, A.R., Blais, D.R., Ghasriani, H., Pezacki, J.P. & Goto, N.K. Activity-based protein profiling of the Escherichia coli GlpG rhomboid protein delineates the catalytic core. Biochemistry 51, 7794–7803 (2012).
Arutyunova, E. et al. Allosteric regulation of rhomboid intramembrane proteolysis. EMBO J. 33, 1869–1881 (2014).
Howarth, M. et al. A monovalent streptavidin with a single femtomolar biotin binding site. Nat. Methods 3, 267–273 (2006).
Klumb, L.A., Chu, V. & Stayton, P.S. Energetic roles of hydrogen bonds at the ureido oxygen binding pocket in the streptavidin-biotin complex. Biochemistry 37, 7657–7663 (1998).
Jeschke, G. DEER distance measurements on proteins. Annu. Rev. Phys. Chem. 63, 419–446 (2012).
Dockter, C. et al. Refolding of the integral membrane protein light-harvesting complex II monitored by pulse EPR. Proc. Natl. Acad. Sci. USA 106, 18485–18490 (2009).
Krishnamani, V., Hegde, B.G., Langen, R. & Lanyi, J.K. Secondary and tertiary structure of bacteriorhodopsin in the SDS denatured state. Biochemistry 51, 1051–1060 (2012).
Hubbell, W.L., Cafiso, D.S. & Altenbach, C. Identifying conformational changes with site-directed spin labeling. Nat. Struct. Biol. 7, 735–739 (2000).
Mok, Y.K., Kay, C.M., Kay, L.E. & Forman-Kay, J. NOE data demonstrating a compact unfolded state for an SH3 domain under non-denaturing conditions. J. Mol. Biol. 289, 619–638 (1999).
Byler, D.M. & Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 25, 469–487 (1986).
Myers, J.K., Pace, C.N. & Scholtz, J.M. Denaturant m values and heat capacity changes: relation to changes in accessible surface areas of protein unfolding. Protein Sci. 4, 2138–2148 (1995).
Renthal, R. An unfolding story of helical transmembrane proteins. Biochemistry 45, 14559–14566 (2006).
Otzen, D.E. Folding of DsbB in mixed micelles: a kinetic analysis of the stability of a bacterial membrane protein. J. Mol. Biol. 330, 641–649 (2003).
Bédard, S., Mayne, L.C., Peterson, R.W., Wand, A.J. & Englander, S.W. The foldon substructure of staphylococcal nuclease. J. Mol. Biol. 376, 1142–1154 (2008).
Llinás, M., Gillespie, B., Dahlquist, F.W. & Marqusee, S. The energetics of T4 lysozyme reveal a hierarchy of conformations. Nat. Struct. Biol. 6, 1072–1078 (1999).
Dutta, A. et al. Characterization of membrane protein non-native states. 2. The SDS-unfolded states of rhodopsin. Biochemistry 49, 6329–6340 (2010).
Lemberg, M.K. & Freeman, M. Functional and evolutionary implications of enhanced genomic analysis of rhomboid intramembrane proteases. Genome Res. 17, 1634–1646 (2007).
Dill, K.A. & Shortle, D. Denatured states of proteins. Annu. Rev. Biochem. 60, 795–825 (1991).
Xue, Y. & Ha, Y. Large lateral movement of transmembrane helix S5 is not required for substrate access to the active site of rhomboid intramembrane protease. J. Biol. Chem. 288, 16645–16654 (2013).
Wu, Z. et al. Structural analysis of a rhomboid family intramembrane protease reveals a gating mechanism for substrate entry. Nat. Struct. Mol. Biol. 13, 1084–1091 (2006).
Liu, T., Whitten, S.T. & Hilser, V.J. Functional residues serve a dominant role in mediating the cooperativity of the protein ensemble. Proc. Natl. Acad. Sci. USA 104, 4347–4352 (2007).
Wang, Y., Maegawa, S., Akiyama, Y. & Ha, Y. The role of L1 loop in the mechanism of rhomboid intramembrane protease GlpG. J. Mol. Biol. 374, 1104–1113 (2007).
Akiyama, Y. & Maegawa, S. Sequence features of substrates required for cleavage by GlpG, an Escherichia coli rhomboid protease. Mol. Microbiol. 64, 1028–1037 (2007).
Hong, H., Chang, Y.C. & Bowie, J.U. Measuring transmembrane helix interaction strengths in lipid bilayers using steric trapping. Methods Mol. Biol. 1063, 37–56 (2013).
Bolen, D.W. & Santoro, M.M. Unfolding free energy changes determined by the linear extrapolation method. 2. Incorporation of delta G degrees N-U values in a thermodynamic cycle. Biochemistry 27, 8069–8074 (1988).
Acknowledgements
This work was supported by startup funds from Michigan State University (H.H.), a Hunt for a Cure grant for basic cystic fibrosis research (H.H.), US National Cancer Institute R01CA149451 (X.H.), US National Eye Institute R01EY005216 (W.L.H.) and the Jules Stein Professor endowment (W.L.H.). The authors thank J. McCracken for general advice on electron paramagnetic resonance (EPR) and for helping with continuous-wave EPR measurements and C. Altenbach for the DEER analysis program. We thank E. Deans and C. Arthur for helping with protein preparations. We also thank R. Jefferson, N. Woodall, Y.-C. Chang, D. Weliky, L. Kroos, J. McCracken and J. Bowie for critical reading of the manuscript. We appreciate suggestions from the anonymous reviewers, which greatly helped improving the quality of this manuscript.
Author information
Authors and Affiliations
Contributions
R.G., K.G., M.K. and H.H. designed experiments, expressed and purified proteins, and performed steric trapping and other biochemical experiments; R.G., X.H. and H.H. designed new steric trapping probes; R.G., S.S. and X.H. synthesized and characterized the new probes; Z.Y. and M.K. performed EPR measurements; Z.Y., M.K., H.H. and W.L.H. interpreted DEER data; All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Table 1, Supplementary Figures 1–14 and Supplementary Note (Synthetic Procedures). (PDF 3217 kb)
Rights and permissions
About this article
Cite this article
Guo, R., Gaffney, K., Yang, Z. et al. Steric trapping reveals a cooperativity network in the intramembrane protease GlpG. Nat Chem Biol 12, 353–360 (2016). https://doi.org/10.1038/nchembio.2048
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2048