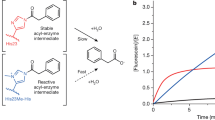
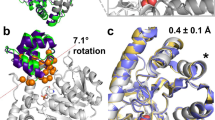
Abstract
A challenge in the computational design of enzymes is that multiple properties, including substrate binding, transition state stabilization and product release, must be simultaneously optimized, and this has limited the absolute activity of successful designs. Here, we focus on a single critical property of many enzymes: the nucleophilicity of an active site residue that initiates catalysis. We design proteins with idealized serine-containing catalytic triads and assess their nucleophilicity directly in native biological systems using activity-based organophosphate probes. Crystal structures of the most successful designs show unprecedented agreement with computational models, including extensive hydrogen bonding networks between the catalytic triad (or quartet) residues, and mutagenesis experiments demonstrate that these networks are critical for serine activation and organophosphate reactivity. Following optimization by yeast display, the designs react with organophosphate probes at rates comparable to natural serine hydrolases. Co-crystal structures with diisopropyl fluorophosphate bound to the serine nucleophile suggest that the designs could provide the basis for a new class of organophosphate capture agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Botos, I. & Wlodawer, A. The expanding diversity of serine hydrolases. Curr. Opin. Struct. Biol. 17, 683–690 (2007).
Hedstrom, L. Serine protease mechanism and specificity. Chem. Rev. 102, 4501–4524 (2002).
Ekici, O.D., Paetzel, M. & Dalbey, R.E. Unconventional serine proteases: variations on the catalytic Ser/His/Asp triad configuration. Protein Sci. 17, 2023–2037 (2008).
Carter, P. & Wells, J.A. Dissecting the catalytic triad of a serine protease. Nature 332, 564–568 (1988).
Corey, D.R. & Craik, C.S. An investigation into the minimum requirements for peptide hydrolysis by mutation of the catalytic triad of trypsin. J. Am. Chem. Soc. 114, 1784–1790 (1992).
Corey, D.R., McGrath, E.M., Vasquez, J.R., Fletterick, R.J. & Craik, C.S. An alternate geometry for the catalytic triad of serine proteases. J. Am. Chem. Soc. 114, 4905–4907 (1992).
Carter, P. & Wells, J.A. Engineering enzyme specificity by “substrate-assisted catalysis”. Science 237, 394–399 (1987).
Simon, G.M. & Cravatt, B.F. Activity-based proteomics of enzyme superfamilies: serine hydrolases as a case study. J. Biol. Chem. 285, 11051–11055 (2010).
Cravatt, B.F., Wright, A.T. & Kozarich, J.W. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu. Rev. Biochem. 77, 383–414 (2008).
Richter, F. et al. Computational design of catalytic dyads and oxyanion holes for ester hydrolysis. J. Am. Chem. Soc. 134, 16197–16206 (2012).
Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010).
Jiang, L. et al. De novo computational design of retro-aldol enzymes. Science 319, 1387–1391 (2008).
Liu, Y., Patricelli, M.P. & Cravatt, B.F. Activity-based protein profiling: the serine hydrolases. Proc. Natl. Acad. Sci. USA 96, 14694–14699 (1999).
Kwasnieski, O., Verdier, L., Malacria, M. & Derat, E. Fixation of the two Tabun isomers in acetylcholinesterase: a QM/MM study. J. Phys. Chem. B 113, 10001–10007 (2009).
Richter, F., Leaver-Fay, A., Khare, S.D., Bjelic, S. & Baker, D. De novo enzyme design using Rosetta3. PLoS ONE 6, e19230 (2011).
Leaver-Fay, A. et al. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol. 487, 545–574 (2011).
Shieh, H.S. et al. Three-dimensional structure of human cytomegalovirus protease. Nature 383, 279–282 (1996).
Chao, G. et al. Isolating and engineering human antibodies using yeast surface display. Nat. Protoc. 1, 755–768 (2006).
Bachovchin, D.A., Brown, S.J., Rosen, H. & Cravatt, B.F. Identification of selective inhibitors of uncharacterized enzymes by high-throughput screening with fluorescent activity-based probes. Nat. Biotechnol. 27, 387–394 (2009).
Labar, G. et al. Crystal structure of the human monoacylglycerol lipase, a key actor in endocannabinoid signaling. ChemBioChem 11, 218–227 (2010).
Schwans, J.P., Sunden, F., Gonzalez, A., Tsai, Y. & Herschlag, D. Evaluating the catalytic contribution from the oxyanion hole in ketosteroid isomerase. J. Am. Chem. Soc. 133, 20052–20055 (2011).
Tsai, P.C. et al. Enzymes for the homeland defense: optimizing phosphotriesterase for the hydrolysis of organophosphate nerve agents. Biochemistry 51, 6463–6475 (2012).
Fischer, S., Arad, A. & Margalit, R. Lipsome-formulated enzymes for organophosphate scavenging: butyrylcholinesterase and Demeton-S. Arch. Biochem. Biophys. 434, 108–115 (2005).
diTargiani, R.C., Chandrasekaran, L., Belinskaya, T. & Saxena, A. In search of a catalytic bioscavenger for the prophylaxis of nerve agent toxicity. Chem. Biol. Interact. 187, 349–354 (2010).
Kim, K., Tsay, O.G., Atwood, D.A. & Churchill, D.G. Destruction and detection of chemical warfare agents. Chem. Rev. 111, 5345–5403 (2011).
Pacsial-Ong, E.J. & Aguilar, Z.P. Chemical warfare agent detection: a review of current trends and future perspective. Front. Biosci. (Schol. Ed.) 5, 516–543 (2013).
Röthlisberger, D. et al. Kemp elimination catalysts by computational enzyme design. Nature 453, 190–195 (2008).
Lenz, D.E. et al. Stoichiometric and catalytic scavengers as protection against nerve agent toxicity: a mini review. Toxicology 233, 31–39 (2007).
Raushel, F.M. Chemical biology: catalytic detoxification. Nature 469, 310–311 (2011).
Smith, A.J. et al. Structural reorganization and preorganization in enzyme active sites: comparisons of experimental and theoretically ideal active site geometries in the multistep serine esterase reaction cycle. J. Am. Chem. Soc. 130, 15361–15373 (2008).
Boström, J., Greenwood, J.R. & Gottfries, J. Assessing the performance of OMEGA with respect to retrieving bioactive conformations. J. Mol. Graph. Model. 21, 449–462 (2003).
Fleishman, S.J., Khare, S.D., Koga, N. & Baker, D. Restricted sidechain plasticity in the structures of native proteins and complexes. Protein Sci. 20, 753–757 (2011).
Rostovtsev, V.V., Green, L.G., Fokin, V.V. & Sharpless, K.B. A stepwise huisgen cycloaddition process: copper(i)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 41, 2596–2599 (2002).
Bachovchin, D.A. et al. Academic cross-fertilization by public screening yields a remarkable class of protein phosphatase methylesterase-1 inhibitors. Proc. Natl. Acad. Sci. USA 108, 6811–6816 (2011).
Eng, J.K., Mccormack, A.L. & Yates, J.R. An approach to correlate tandem mass-spectral data of peptides with amino-acid-sequences in a protein database. J. Am. Soc. Mass Spectrom. 5, 976–989 (1994).
Luft, J.R. et al. A deliberate approach to screening for initial crystallization conditions of biological macromolecules. J. Struct. Biol. 142, 170–179 (2003).
Zbyszek Otwinowski, W.M. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Long, F., Vagin, A.A., Young, P. & Murshudov, G.N. BALBES: a molecular-replacement pipeline. Acta Crystallogr. D Biol. Crystallogr. 64, 125–132 (2008).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Winn, M.D., Isupov, M.N. & Murshudov, G.N. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D Biol. Crystallogr. 57, 122–133 (2001).
Acknowledgements
We thank K. Masuda and D. Milliken for helping with ABPP screening, R. Xiao and G. Kornhaber for experimental support with sample preparation and structure determination and the Rosen Lab for sharing instrumentation for performing fluopol experiments. This work was supported in part by the Defense Threat Reduction Agency (DTRA) (D.B.), the National Institute on Drug Abuse grant DA033670 (B.F.C.) and by a grant from the National Institute of General Medical Sciences Protein Structure Initiative (PSI), U54-GM094597 (J.F.H.). Fellowship support from the Sir Henry Wellcome Postdoctoral Fellowship (S.R.), NIH–National Institute of Environmental Health Sciences K99/R00 Pathways to Independence Postdoctoral Award 1K99ES020851-01 (C.W.) and Helen Hay Whitney Fellowship (M.L.M.) are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
S.R., C.W., B.F.C. and D.B. conceived the project. S.R. and F.R. performed the computational design, S.R. expressed and purified the designed proteins. S.R. and K.Y. performed the yeast display experiments. C.W. performed the ABPP screening and mass spec experiments. C.W. and M.L.M. performed the fluopol experiments. A.P.K., A.E.M., S.L., J.S., M.S. and J.F.H. performed the crystallography experiments. S.R., C.W., B.F.C. and D.B. analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–17 and Supplementary Tables 1–5. (PDF 3483 kb)
Rights and permissions
About this article
Cite this article
Rajagopalan, S., Wang, C., Yu, K. et al. Design of activated serine–containing catalytic triads with atomic-level accuracy. Nat Chem Biol 10, 386–391 (2014). https://doi.org/10.1038/nchembio.1498
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1498
This article is cited by
-
Mce1R of Mycobacterium tuberculosis prefers long-chain fatty acids as specific ligands: a computational study
Molecular Diversity (2023)
-
Design and evolution of an enzyme with a non-canonical organocatalytic mechanism
Nature (2019)
-
Computational redesign of enzymes for regio- and enantioselective hydroamination
Nature Chemical Biology (2018)
-
Emergence of a catalytic tetrad during evolution of a highly active artificial aldolase
Nature Chemistry (2017)
-
Functional Frankensteins
Nature Chemistry (2016)