Abstract
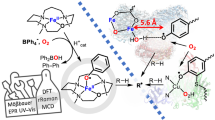
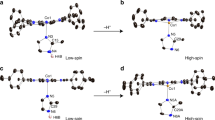
Strong electron-donation from the axial thiolate ligand of cytochrome P450 has been proposed to increase the reactivity of compound I with respect to C–H bond activation. However, it has proven difficult to test this hypothesis, and a direct link between reactivity and electron donation has yet to be established. To make this connection, we have prepared a selenolate-ligated cytochrome P450 compound I intermediate. This isoelectronic perturbation allows for direct comparisons with the wild-type enzyme. Selenium incorporation was achieved using a cysteine auxotrophic Escherichia coli strain. The intermediate was prepared with meta-chloroperbenzoic acid and characterized by UV–visible, Mössbauer and electron paramagnetic resonance spectroscopies. Measurements revealed increased asymmetry around the ferryl moiety, consistent with increased electron donation from the axial selenolate ligand. In line with this observation, we find that the selenolate-ligated compound I cleaves C–H bonds more rapidly than the wild-type intermediate.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rittle, J. & Green, M. T. Cytochrome P450 compound I: capture, characterization, and C-H bond activation kinetics. Science 330, 933–937 (2010).
Yosca, T. H. et al. Iron(IV)hydroxide pKa and the role of thiolate ligation in C–H bond activation by cytochrome P450. Science 342, 825–829 (2013).
Dunford, H. B. Heme Peroxidases (Wiley-VCH, 1999).
Peter, S. et al. Selective hydroxylation of alkanes by an extracellular fungal peroxygenase. FEBS J. 278, 3667–3675 (2011).
Wang, X., Peter, S., Kinne, M., Hofrichter, M. & Groves, J. T. Detection and kinetic characterization of a highly reactive heme-thiolate peroxygenase compound I. J. Am. Chem. Soc. 134, 12897–12900 (2012).
Hu, S. H. & Hager, L. P. Highly enantioselective propargylic hydroxylations catalyzed by chloroperoxidase. J. Am. Chem. Soc. 121, 872–873 (1999).
Zaks, A. & Dodds, D. R. Chloroperoxidase-catalyzed asymmetric oxidations: substrate specificity and mechanistic study. J. Am. Chem. Soc. 117, 10419–10424 (1995).
Yosca, T. H. et al. Setting an upper limit on the myoglobin iron(IV)hydroxide pKa: insight into axial ligand tuning in heme protein catalysis. J. Am. Chem. Soc. 136, 9124–9131 (2014).
Green, M. T., Dawson, J. H. & Gray, H. B. Oxoiron(IV) in chloroperoxidase compound II is basic: implications for P450 chemistry. Science 304, 1653–1656 (2004).
Wang, X., Ullrich, R., Hofrichter, M. & Grove, J. T. Heme-thiolate ferryl of aromatic peroxygenase is basic and reactive. Proc. Natl Acad. Sci. USA 112, 3686–3691 (2015).
Krest, C. M. et al. Significantly shorter Fe–S bond in cytochrome P450-I is consistent with greater reactivity relative to chloroperoxidase. Nat. Chem. 7, 696–702 (2015).
Takahashi, A., Kurahashi, T. & Fujii, H. Effect of imidazole and phenolate axial ligands on the electronic structure and reactivity of oxoiron(IV) porphyrin π-cation radical complexes: drastic increase in oxo-transfer and hydrogen abstraction reactivities. Inorg. Chem. 48, 2614–2625 (2009).
Prokop, K. A., de Visser, S. P. & Goldberg, D. P. Unprecedented rate enhancements of hydrogen-atom transfer to a manganese(V)-oxo corrolazine complex. Angew. Chem. Int. Ed. 49, 5091–5095 (2010).
Gross, Z. & Nimri, S. A pronounced axial ligand effect on the reactivity of oxoiron(IV) porphyrin cation radicals. Inorg. Chem. 33, 1731–1732 (1994).
Ohno, T. et al. Remarkable axial thiolate ligand effect on the oxidation of hydrocarbons by active intermediate of iron porphyrin and cytochrome P450. J. Inorg. Biochem. 82, 123–125 (2000).
Adachi, S., Nagano, S., Watanabe, Y., Ishimori, K. & Morishima, I. Alteration of human myoglobin proximal histidine to cysteine or tyrosine by site-directed mutagenesis: characterization and their catalytic activities. Biochem. Biophys. Res. Commun. 180, 138–144 (1991).
Adachi, S. et al. Roles of proximal ligand in heme-proteins: replacement of proximal histidine of human myoglobin with cysteine and tyrosine by site-directed mutagenesis as models for P-450, chloroperoxidase, and catalase. Biochemistry 32, 241–252 (1993).
Hildebrand, D. P., Ferrer, J. C., Tang, H. L., Smith, M. & Mauk, A. G. Trans effects on cysteine ligation in the proximal his93cys variant of horse heart myoglobin. Biochemistry 34, 11598–11605 (1995).
Sigman, J. A., Pond, A. E., Dawson, J. H. & Lu, Y. Engineering cytochrome c peroxidase into cytochrome P450: a proximal effect on heme-thiolate ligation. Biochemistry 38, 11122–11129 (1999).
Murugan, R. & Mazumdar, S. Structure and redox properties of the haem centre on the C357M mutant of cytochrome P450cam. ChemBioChem 6, 1204–1211 (2005).
Yoshioka, S., Takahashi, S., Hori, H., Ishimori, K. & Morishima, I. Proximal cysteine residue is essential for the enzymatic activities of cytochrome P450cam . Eur. J. Biochem. 268, 252–259 (2001).
Auclair, K., Moënne-Loccoz, P. & de Montellano, P. R. O. Roles of the proximal heme thiolate ligand in cytochrome P450cam . J. Am. Chem. Soc. 123, 4877–4885 (2001).
Coelho, P. S. et al. A serine-substituted P450 catalyzes highly efficient carbene transfer to olefins in vivo. Nat. Chem. Biol. 9, 485–487 (2013).
Vatsis, K. P., Peng, H. M. & Coon, M. J. Replacement of active-site cysteine-436 by serine converts cytochrome P450 2B4 into an NADPH oxidase with negligible monooxygenase activity. J. Inorg. Biochem. 91, 542–553 (2002).
Vatsis, K. P., Peng, H. M. & Coon, M. J. Abolition of oxygenase function, retention of NADPH oxidase activity, and emergence of peroxidase activity upon replacement of the axial cysteine-436 ligand by histidine in cytochrome P450 2B4. Arch. Biochem. Biophys. 434, 128–138 (2005).
Moroder, L. Isosteric replacement of sulfur with other chalcogens in peptides and proteins. J. Pept. Sci. 11, 187–214 (2005).
Huber, R. E. & Criddle, R. S. Comparison of chemical properties of selenocysteine and selenocystine with their sulfur analogs. Arch. Biochem. Biophys. 122, 164–173 (1967).
Jiang, Y. Y. et al. Calculated and experimental spin state of seleno cytochrome P450. Angew. Chem. Int. Ed. 48, 7193–7195 (2009).
Aldag, C. et al. Probing the role of the proximal heme ligand in cytochrome P450cam by recombinant incorporation of selenocysteine. Proc. Natl Acad. Sci. USA 106, 5481–5486 (2009).
Sivaramakrishnan, S. et al. A novel intermediate in the reaction of seleno CYP119 with m-chloroperbenzoic acid. Biochemistry 50, 3014–3024 (2011).
Sivaramakrishnan, S. et al. Proximal ligand electron donation and reactivity of the cytochrome P450 ferric-peroxo anion. J. Am. Chem. Soc. 134, 6673–6684 (2012).
Vandemeulebroucke, A., Aldag, C., Stiebritz, M. T., Reiher, M. & Hilvert, D. Kinetic consequences of introducing a proximal selenocysteine ligand into cytochrome P450cam. Biochemistry 54, 6692–6703 (2015).
Cohen, S., Kumar, D. & Shaik, S. In silico design of a mutant of cytochrome P450 containing selenocysteine. J. Am. Chem. Soc. 128, 2649–2653 (2006).
Yosca, T. H. & Green, M. T. Preparation of compound I in P450cam: the prototypical P450. Isr. J. Chem. 56, 834–840 (2016).
Müller, S. et al. The formation of diselenide bridges in proteins by incorporation of selenocysteine residues: biosynthesis and characterization of (Se)2-thioredoxin. Biochemistry 33, 3404–3412 (1994).
Sanchez, J. F., Hoh, F., Strub, M. P., Aumelas, A. & Dumas, C. Structure of the cathelicidin motif of protegrin-3 precursor: structural insights into the activation mechanism of an antimicrobial protein. Structure 10, 1363–1370 (2002).
Strub, M. P. et al. Selenomethionine and selenocysteine double labeling strategy for crystallographic phasing. Structure 11, 1359–1367 (2003).
Rutter, R. et al. Chloroperoxidase compound-I: electron paramagnetic resonance and Mössbauer studies. Biochemistry 23, 6809–6816 (1984).
Rittle, J., Younker, J. M. & Green, M. T. Cytochrome P450: the active oxidant and its spectrum. Inorg. Chem. 49, 3610–3617 (2010).
Groves, J. T., McClusky, G. A., White, R. E. & Coon, M. J. Aliphatic hydroxylation by highly purified liver microsomal cytochrome P-450. evidence for a carbon radical intermediate. Biochem. Biophys. Res. Commun. 81, 154–160 (1978).
Mayer, J. M. Hydrogen atom abstraction by metal-oxo complexes: understanding the analogy with organic radical reactions. Acc. Chem. Res. 31, 441–450 (1998).
Green, M. T. C–H bond activation in heme proteins the role of thiolate ligation in cytochrome P450. Curr. Opin. Chem. Biol. 13, 84–88 (2009).
Epel, B. & Silakov, A. Kazan Viewer Version 2.1 (2006); https://sites.google.com/site/silakovalexey/kazan-viewer
Stoll, S. & Schweiger, A. Easyspin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 178, 42–55 (2006).
Acknowledgements
This work was supported by NIH (R01-GM101390).
Author information
Authors and Affiliations
Contributions
E.O. and M.G. wrote the manuscript and designed the experiments. E.O. prepared the samples, collected and analysed stopped-flow, Mössbauer and EPR data. A.S. collected and analysed EPR data. T.Y. provided input on and assisted with experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 964 kb)
Rights and permissions
About this article
Cite this article
Onderko, E., Silakov, A., Yosca, T. et al. Characterization of a selenocysteine-ligated P450 compound I reveals direct link between electron donation and reactivity. Nature Chem 9, 623–628 (2017). https://doi.org/10.1038/nchem.2781
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2781
This article is cited by
-
Photothermal-enabled single-atom catalysts for high-efficiency hydrogen peroxide photosynthesis from natural seawater
Nature Communications (2023)
-
Custom selenoprotein production enabled by laboratory evolution of recoded bacterial strains
Nature Biotechnology (2018)
-
New functional twists for P450s
Nature Chemistry (2017)