Abstract
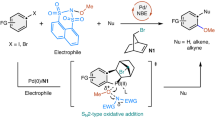
Arylmetals are highly valuable carbon nucleophiles that are readily and inexpensively prepared from aryl halides or arenes and widely used on both laboratory and industrial scales to react directly with a wide range of electrophiles. Although C−C bond formation has been a staple of organic synthesis, the direct transfer of primary amino (−NH2) and hydroxyl (−OH) groups to arylmetals in a scalable and environmentally friendly fashion remains a formidable synthetic challenge because of the absence of suitable heteroatom-transfer reagents. Here, we demonstrate the use of bench-stable N−H and N−alkyl oxaziridines derived from readily available terpenoid scaffolds as efficient multifunctional reagents for the direct primary amination and hydroxylation of structurally diverse aryl- and heteroarylmetals. This practical and scalable method provides one-step synthetic access to primary anilines and phenols at low temperature and avoids the use of transition-metal catalysts, ligands and additives, nitrogen-protecting groups, excess reagents and harsh workup conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rappoport, Z. The Chemistry of Phenols (John Wiley & Sons, 2004).
Rappoport, Z. The Chemistry of Anilines, Parts 1–2 (John Wiley & Sons, 2007).
Blaser, H.-U., Steiner, H. & Studer, M. Selective catalytic hydrogenation of functionalized nitroarenes: an update. ChemCatChem 1, 210–221 (2009).
Klinkenberg, J. L. & Hartwig, J. F. Catalytic organometallic reactions of ammonia. Angew. Chem. Int. Ed. 50, 86–95 (2011).
Qiao, J. X. & Lam, P. Y. S. in Boronic Acids 2nd edn, Vol. 1 (ed. Hall, D. G.) 315–361 (Wiley-VCH, 2011).
Wolfe, J. P., Wagaw, S., Marcoux, J.-F. & Buchwald, S. L. Rational development of practical catalysts for aromatic carbon–nitrogen bond formation. Acc. Chem. Res. 31, 805–818 (1998).
Vo, G. D. & Hartwig, J. F. Palladium-catalyzed coupling of ammonia with aryl chlorides, bromides, iodides, and sulfonates: a general method for the preparation of primary arylamines. J. Am. Chem. Soc. 131, 11049–11061 (2009).
Barker, T. J. & Jarvo, E. R. Umpolung amination: nickel-catalyzed coupling reactions of N,N-dialkyl-N-chloroamines with diorganozinc reagents. J. Am. Chem. Soc. 131, 15598–15599 (2009).
Rucker, R. P., Whittaker, A. M., Dang, H. & Lalic, G. Synthesis of hindered anilines: copper-catalyzed electrophilic amination of aryl boronic esters. Angew. Chem. Int. Ed. 51, 3953–3956 (2012).
Berman, A. M. & Johnson, J. S. Copper-catalyzed electrophilic amination of diorganozinc reagents. J. Am. Chem. Soc. 126, 5680–5681 (2004).
Barker, T. J. & Jarvo, E. R. Developments in transition-metal-catalyzed reactions using electrophilic nitrogen sources. Synthesis 3954–3964 (2011).
Mąkosza, M. Nucleophilic substitution of hydrogen in electron-deficient arenes, a general process of great practical value. Chem. Soc. Rev. 39, 2855–2868 (2010).
Makosza, M. Reactions of nucleophiles with nitroarenes: multifacial and versatile electrophiles. Chem. Eur. J. 20, 5536–5545 (2014).
Terrier, F. Modern Nucleophilic Aromatic Substitution (John Wiley & Sons, 2013).
Alvarez-Builla, J., Vaquero, J. J. & Barlueng, J. Modern Heterocyclic Chemistry (John Wiley & Sons, 2011).
Yoo, E. J., Ma, S., Mei, T.-S., Chan, K. S. L. & Yu, J.-Q. Pd-catalyzed intermolecular C–H amination with alkylamines. J. Am. Chem. Soc. 133, 7652–7655 (2011).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C–H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Chinnusamy, T., Feeney, K., Watson, C. G., Leonori, D. & Aggarwal, V. K. in Comprehensive Organic Synthesis 2nd edn, Vol. 7 (eds Knockel, P. & Molander, G. A.) 692–718 (Elsevier, 2014).
Enthaler, S. & Company, A. Palladium-catalyzed hydroxylation and alkoxylation. Chem. Soc. Rev. 40, 4912–4924 (2011).
Alonso, D. A., Najera, C., Pastor, I. M. & Yus, M. Transition-metal-catalyzed synthesis of hydroxylated arenes. Chem. Eur. J. 16, 5274–5284 (2010).
Garrett, C. E. & Prasad, K. The art of meeting palladium specifications in active pharmaceutical ingredients produced by Pd-catalyzed reactions. Adv. Synth. Catal. 346, 889–900 (2004).
Knochel, P. et al. Highly functionalized organomagnesium reagents prepared through halogen–metal exchange. Angew. Chem. Int. Ed. 42, 4302–4320 (2003).
Klatt, T., Markiewicz, J. T., Sämann, C. & Knochel, P. Strategies to prepare and use functionalized organometallic reagents. J. Org. Chem. 79, 4253–4269 (2014).
Rappoport, Z. & Marek, I. The Chemistry of Organomagnesium Compounds (John Wiley & Sons, 2008).
Erdik, E. in The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids (ed. Patai, S.) 303–341 (Wiley, 2009).
Corpet, M. & Gosmini, C. Recent advances in electrophilic amination reactions. Synthesis 46, 2258–2271 (2014).
Starkov, P., Jamison, T. F. & Marek, I. Electrophilic amination: the case of nitrenoids. Chem. Eur. J. 21, 5278–5300 (2015).
Kitamura, M., Suga, T., Chiba, S. & Narasaka, K. Synthesis of primary amines by the electrophilic amination of Grignard reagents with 1,3-dioxolan-2-one O-sulfonyloxime. Org. Lett. 6, 4619–4621 (2004).
Kitamura, M., Chiba, S. & Narasaka, K. Synthesis of primary amines and N-methylamines by the electrophilic amination of Grignard reagents with 2-imidazolidinone O-sulfonyloxime. Bull. Chem. Soc. Jpn 76, 1063–1070 (2003).
Tsutsui, H., Ichikawa, T. & Narasaka, K. Preparation of primary amines by the alkylation of O-sulfonyloximes of benzophenone derivatives with Grignard reagents. Bull. Chem. Soc. Jpn 72, 1869–1878 (1999).
Chiba, S. & Narasaka, K. in Amino Group Chemistry (ed. Ricci, A.) 1–54 (Wiley VCH, 2008).
Zhu, C., Li, G., Ess, D. H., Falck, J. R. & Kürti, L. Elusive metal-free primary amination of arylboronic acids: synthetic studies and mechanism by density functional theory. J. Am. Chem. Soc. 134, 18253–18256 (2012).
Mlynarski, S. N., Karns, A. S. & Morken, J. P. Direct stereospecific amination of alkyl and aryl pinacol boronates. J. Am. Chem. Soc. 134, 16449–16451 (2012).
Davis, F. A., Mancinelli, P. A., Balasubramanian, K. & Nadir, U. K. Coupling and hydroxylation of lithium and Grignard reagents by oxaziridines. J. Am. Chem. Soc. 101, 1044–1045 (1979).
Davis, F. A., Wei, J., Sheppard, A. C. & Gubernick, S. The mechanism of hydroxylation of organometallic reagents by 2-sulfonyloxaziridines. Tetrahedron Lett. 28, 5115–5118 (1987).
He, Z. & Jamison, T. F. Continuous-flow synthesis of functionalized phenols by aerobic oxidation of Grignard reagents. Angew. Chem. Int. Ed. 53, 3353–3357 (2014).
Corey, E. J. & Gross, A. W. Methods for the synthesis of chiral hindered amines. J. Org. Chem. 50, 5391–5393 (1985).
Blanc, S., Bordogna, C. A. C., Buckley, B. R., Elsegood, M. R. J. & Page, P. C. B. New stable N–H oxaziridines—synthesis and reactivity. Eur. J. Org. Chem. 2010, 882–889 (2010).
Page, P. C. B., Limousin, C. & Murrell, V. L. Asymmetric electrophilic amination of various carbon nucleophiles with enantiomerically pure chiral N–H oxaziridines derived from camphor and fenchone. J. Org. Chem. 67, 7787–7796 (2002).
Page, P. C. B. et al. The first stable enantiomerically pure chiral N–H oxaziridines: synthesis and reactivity. J. Org. Chem. 65, 4204–4207 (2000).
Choong, I. C. & Ellman, J. A. Synthesis of alkoxylamines by alkoxide amination with 3,3′-di-tert-butyloxaziridine. J. Org. Chem. 64, 6528–6529 (1999).
Williamson, K. S., Michaelis, D. J. & Yoon, T. P. Advances in the chemistry of oxaziridines. Chem. Rev. 114, 8016–8036 (2014).
Davis, F. A., Chen, B.-C. & Zhou, P. in Comprehensive Heterocyclic Chemistry III. Vol. 1A (ed. Katritzky, A. R.) 559–622 (Elsevier, 2008).
Mori, T. & Kato, S. Analytical RISM-MP2 free energy gradient method: application to the Schlenk equilibrium of Grignard reagent. Chem. Phys. Lett. 437, 159–163 (2007).
Haines, B. E. & Wiest, O. SET-induced biaryl cross-coupling: an SRN1 reaction. J. Org. Chem. 79, 2771–2774 (2014).
Otte, D. A. L. & Woerpel, K. A. Evidence that additions of Grignard reagents to aliphatic aldehydes do not involve single-electron-transfer processes. Org. Lett. 17, 3906–3909 (2015).
Aminova, R. M. & Ermakova, E. Rearrangements and proton transfer in nitrones by quantum chemistry and molecular dynamics. Chem. Phys. Lett. 359, 184–190 (2002).
Jiménez-Osés, G., Brockway, A. J., Shaw, J. T. & Houk, K. N. Mechanism of alkoxy groups substitution by Grignard reagents on aromatic rings and experimental verification of theoretical predictions of anomalous reactions. J. Am. Chem. Soc. 135, 6633–6642 (2013).
Chai, J.-D. & Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620 (2008).
Acknowledgements
L.K. gratefully acknowledges the generous financial support from Rice University, National Institutes of Health (R01 GM-114609-01), National Science Foundation (CAREER:SusChEM CHE-1455335), the Robert A. Welch Foundation (Grant C-1764), Amgen (2014 Young Investigators' Award) and Biotage (2015 Young Principal Investigator Award), which are greatly appreciated. D.H.E. thanks BYU and the Fulton Supercomputing Lab. We thank S. Sutton and P. Richardson at Pfizer LaJolla for the thorough DSC analysis of the oxaziridine reagents used in this manuscript—the full DSC analysis and interpretation data are included in the Supplementary Information. We also thank E. M. Carreira, A. J. Catino, E.J. Corey, F. A. Davis, A. Ganesan, M. M. Joullie, J. Lopchuk, I. Marek, A. G. Myers, K.C. Nicolaou, J. Njardarson, A. Padwa, R. Sarpong, A. Toro and E. Vedejs for helpful commentary. We dedicate this article to Professor K. C. Nicolaou on the occasion of his 70th birthday.
Author information
Authors and Affiliations
Contributions
H.G. and L.K. conceived this work; H.G., Z.Z. and L.K. designed the organic chemistry experiments; H.G., Z.Z. and N.E.B. conducted the organic chemistry experiments and analysed the data; D.-H.K., D.H.E. and L.K. designed the computational studies; D.-H. K., J.C., S.J. and D.H.E. conducted the calculations and analysed the data; D.H.E. and L.K. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 28929 kb)
Rights and permissions
About this article
Cite this article
Gao, H., Zhou, Z., Kwon, DH. et al. Rapid heteroatom transfer to arylmetals utilizing multifunctional reagent scaffolds. Nature Chem 9, 681–688 (2017). https://doi.org/10.1038/nchem.2672
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2672
This article is cited by
-
Camphor nitroimine: a key building block in unusual transformations and its applications in the synthesis of bioactive compounds
Molecular Diversity (2022)
-
Room-temperature copper-catalyzed electrophilic amination of arylcadmium iodides with ketoximes
Journal of the Iranian Chemical Society (2021)
-
Organocatalytic nitrogen transfer to unactivated olefins via transient oxaziridines
Nature Catalysis (2020)
-
From alkylarenes to anilines via site-directed carbon–carbon amination
Nature Chemistry (2019)