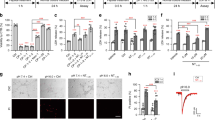
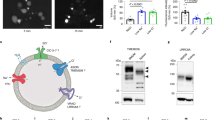
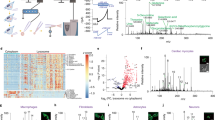
Abstract
Perturbations in cellular chloride concentrations can affect cellular pH and autophagy and lead to the onset of apoptosis. With this in mind, synthetic ion transporters have been used to disturb cellular ion homeostasis and thereby induce cell death; however, it is not clear whether synthetic ion transporters can also be used to disrupt autophagy. Here, we show that squaramide-based ion transporters enhance the transport of chloride anions in liposomal models and promote sodium chloride influx into the cytosol. Liposomal and cellular transport activity of the squaramides is shown to correlate with cell death activity, which is attributed to caspase-dependent apoptosis. One ion transporter was also shown to cause additional changes in lysosomal pH, which leads to impairment of lysosomal enzyme activity and disruption of autophagic processes. This disruption is independent of the initiation of apoptosis by the ion transporter. This study provides the first experimental evidence that synthetic ion transporters can disrupt both autophagy and induce apoptosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell 147, 728–741 (2011).
Ohsumi, Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat. Rev. Mol. Cell Biol. 2, 211–216 (2001).
Kuma, A. et al. The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036 (2004).
White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 12, 401–410 (2012).
Baek, K.-H., Park, J. & Shin, I. Autophagy-regulating small molecules and their therapeutic applications. Chem. Soc. Rev. 41, 3245–3263 (2012).
Newmeyer, D. D. & Ferguson-Miller, S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112, 481–490 (2003).
Igney, F. H. & Krammer, P. H. Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer 2, 277–288 (2002).
Williams, D. R., Ko, S.-K., Park, S., Lee, M.-R. & Shin, I. An apoptosis-inducing small molecule that binds to heat shock protein 70. Angew. Chem. Int. Ed. 47, 7466–7469 (2008).
Ko, S.-K. et al. A small molecule inhibitor of ATPase activity of HSP70 induces apoptosis and has antitumor activities. Chem. Biol. 22, 391–403 (2015).
Ko, S.-K. et al. Synthetic ion transporters can induce apoptosis by facilitating chloride anion transport into cells. Nat. Chem. 6, 885–892 (2014).
Stauber, T. & Jentsch, T. J. Chloride in vesicular trafficking and function. Annu. Rev. Physiol. 75, 453–477 (2013).
Gould, D. Human physiology: From cells to systems, 3rd edition. J. Adv. Nurs. 28, 680–682 (1998).
Yu, S. P., Canzoniero, L. M. T. & Choi, D. W. Ion homeostasis and apoptosis. Curr. Opin. Cell Biol. 13, 405–411 (2001).
Hosogi, S., Kusuzaki, K., Inui, T., Wang, X. & Marunaka, Y. Cytosolic chloride ion is a key factor in lysosomal acidification and function of autophagy in human gastric cancer cell. J. Cell. Mol. Med. 18, 1124–1133 (2014).
Anke, D. et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol. 8, 933–944 (2006).
Sessler, J. L. et al. Synthesis, anion-binding properties, and in vitro anticancer activity of prodigiosin analogues. Angew. Chem. Int. Ed. 117, 6143–6146 (2005).
Ohkuma, S. et al. Prodigiosins uncouple lysosomal vacuolar-type ATPase through promotion of H+/Cl− symport. Biochem. J. 334, 731–741 (1998).
Melvin, M. S. et al. Double-strand DNA cleavage by copper·prodigiosin. J. Am. Chem. Soc. 122, 6333–6334 (2000).
Li, H. et al. Efficient, non-toxic anion transport by synthetic carriers in cells and epithelia. Nat. Chem. 8, 24–32 (2016).
Busschaert, N. et al. Squaramides as potent transmembrane anion transporters. Angew. Chem. Int. Ed. 51, 4426–4430 (2012).
Jin, C. et al. Squaramide-based tripodal receptors for selective recognition of sulfate anion. Chem. Commun. 49, 2025–2027 (2013).
Busschaert, N. et al. Towards predictable transmembrane transport: QSAR analysis of anion binding and transport. Chem. Sci. 4, 3036–3045 (2013).
Busschaert, N. et al. Structure–activity relationships in tripodal transmembrane anion transporters: the effect of fluorination. J. Am. Chem. Soc. 133, 14136–14148 (2011).
Moore, S. J. et al. Chloride, carboxylate and carbonate transport by ortho-phenylenediamine-based bisureas. Chem. Sci. 4, 103–117 (2013).
Clement, N. R. & Gould, J. M. Pyranine (8-hydroxy-1,3,6-pyrenetrisulfonate) as a probe of internal aqueous hydrogen ion concentration in phospholipid vesicles. Biochemistry 20, 1534–1538 (1981).
Busschaert, N. et al. Thiosquaramides: pH switchable anion transporters. Chem. Sci. 5, 3617–3626 (2014).
Valkenier, H., Haynes, C. J. E., Herniman, J., Gale, P. A. & Davis, A. P. Lipophilic balance – a new design principle for transmembrane anion carriers. Chem. Sci. 5, 1128–1134 (2014).
Li, Z., Deng, L.-Q., Chen, J.-X., Zhou, C.-Q. & Chen, W.-H. Does lipophilicity affect the effectiveness of a transmembrane anion transporter? Insight from squaramido-functionalized bis(choloyl) conjugates. Org. Biomol. Chem. 13, 11761–11769 (2015).
Hagelin, H., Murray, J. S., Politzer, P., Brinck, T. & Berthelot, M. Family-independent relationships between computed molecular surface quantities and solute hydrogen bond acidity/basicity and solute-induced methanol O–H infrared frequency shifts. Can. J. Chem. 73, 483–488 (1995).
Behr, J. P., Kirch, M. & Lehn, J. M. Carrier-mediated transport through bulk liquid membranes: dependence of transport rates and selectivity on carrier properties in a diffusion-limited process. J. Am. Chem. Soc. 107, 241–246 (1985).
Vargas Jentzsch, A. et al. Ditopic ion transport systems: anion–π interactions and halogen bonds at work. Angew. Chem. Int. Ed. 50, 11675–11678 (2011).
Wu, X. et al. Nonprotonophoric electrogenic chloride transport mediated by valinomycin-like carriers. Chem 1, 127–146 (2016).
Howe, E. N. W. et al. pH-regulated nonelectrogenic anion transport by phenylthiosemicarbazones. J. Am. Chem. Soc. 138, 8301–8308 (2016).
Läuger, P. Mechanisms of biological ion transport—carriers, channels, and pumps in artificial lipid membranes. Angew. Chem. Int. Ed. 24, 905–923 (1985).
Mollenhauer, H. H., James Morré, D. & Rowe, L. D. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim. Biophys. Acta 1031, 225–246 (1990).
Tsukimoto, M., Harada, H., Ikari, A. & Takagi, K. Involvement of chloride in apoptotic cell death induced by activation of ATP-sensitive P2 × 7 purinoceptor. J. Biol. Chem. 280, 2653–2658 (2005).
Bortner, C. D. & Cidlowski, J. A. Uncoupling cell shrinkage from apoptosis reveals that Na+ influx is required for volume loss during programmed cell death. J. Biol. Chem. 278, 39176–39184 (2003).
Saha, T., Hossain, M. S., Saha, D., Lahiri, M. & Talukdar, P. Chloride-mediated apoptosis-inducing activity of bis(sulfonamide) anionophores. J. Am. Chem. Soc. 138, 7558–7567 (2016).
Waterhouse, N. J. et al. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J. Cell Biol. 153, 319–328 (2001).
Tzung, S.-P. et al. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat. Cell Biol. 3, 183–191 (2001).
Elmore, S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35, 495–516 (2007).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997).
Joza, N. et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410, 549–554 (2001).
Cho, H. J. et al. A small molecule that binds to an ATPase domain of Hsc70 promotes membrane trafficking of mutant cystic fibrosis transmembrane conductance regulator. J. Am. Chem. Soc. 133, 20267–20276 (2011).
Perelman, A. et al. JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 3, e430 (2012).
Yang, Y.-P. et al. Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol. Sin. 34, 625–635 (2013).
Xie, Z. & Klionsky, D. J. Autophagosome formation: core machinery and adaptations. Nat. Cell Biol. 9, 1102–1109 (2007).
Graves, A. R., Curran, P. K., Smith, C. L. & Mindell, J. A. The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 453, 788–792 (2008).
DiCiccio, J. E. & Steinberg, B. E. Lysosomal pH and analysis of the counter ion pathways that support acidification. J. Gen. Physiol. 137, 385–390 (2011).
Creasy, B. M., Hartmann, C. B., White, F. K. H. & McCoy, K. L. New assay using fluorogenic substrates and immunofluorescence staining to measure cysteine cathepsin activity in live cell subpopulations. Cytometry Part A 71A, 114–123 (2007).
Kimura, S., Noda, T. & Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3, 452–460 (2007).
Chen, Y., McMillan-Ward, E., Kong, J., Israels, S. J. & Gibson, S. B. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 15, 171–182 (2007).
Jahreiss, L., Menzies, F. M. & Rubinsztein, D. C. The itinerary of autophagosomes: from peripheral formation to kiss-and-run fusion with lysosomes. Traffic 9, 574–587 (2008).
Acknowledgements
This study was supported financially by the National Creative Research Initiative (grant no. 2010-0018272 to I.S.) and Basic Science Research programmes (grant no. NRF-2012R1A1A1040142 to W.N.) in Korea. P.A.G. acknowledges the EPSRC for postdoctoral fellowships (EP/J009687/1 to N.B. and E.N.W.H.) and the Royal Society and the Wolfson Foundation for a Research Merit Award. The work in Austin was supported by the National Institutes of Health (grant no. GM103790 to J.L.S.). The theoretical studies were supported by projects P2020-PTDC/QEQ-SUP/4283/2014, CICECO – Aveiro Institute of Materials (UID/CTM/50011/2013) and iBiMED – Institute of Biomedicine (UID/BIM/04501/2013), financed by National Funds through the FCT/MEC and co-financed by QREN-FEDER through COMPETE under the PT2020 Partnership Agreement. I.M. acknowledges the FCT for PhD scholarship SFRH/BD/87520/2012. The authors thank H.J. Clarke for resynthesizing compound 5.
Author information
Authors and Affiliations
Contributions
I.S. and N.B. designed the study and wrote the manuscript. J.L.S. and P.A.G. helped with writing the manuscript. I.S., J.L.S and P.A.G. supervised the project. S.-H.P., K.-H.B. and Y.P.C. performed biological studies. N.B. designed, synthesized and characterized the compounds and performed the ion-transport studies in liposomes and the anion binding studies. E.N.W.H., J.R.H. and L.E.K. helped with some of the liposomal studies. I.M. and V.F. carried out DFT calculations and Vs,max calculations. J.P. and W.N. carried out ion-transport activity studies in cells.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5271 kb)
Supplementary information
Crystallographic data for compound 5a. (CIF 565 kb)
Supplementary information
Crystallographic data for compound 5b. (CIF 1015 kb)
Supplementary information
Crystallographic data for compound 8. (CIF 752 kb)
Rights and permissions
About this article
Cite this article
Busschaert, N., Park, SH., Baek, KH. et al. A synthetic ion transporter that disrupts autophagy and induces apoptosis by perturbing cellular chloride concentrations. Nature Chem 9, 667–675 (2017). https://doi.org/10.1038/nchem.2706
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2706
This article is cited by
-
In vivo therapy of osteosarcoma using anion transporters-based supramolecular drugs
Journal of Nanobiotechnology (2024)
-
Squaramides enhance NLRP3 inflammasome activation by lowering intracellular potassium
Cell Death Discovery (2023)
-
Measuring anion binding at biomembrane interfaces
Nature Communications (2022)
-
Ion transporters: emerging agents for anticancer therapy
Science China Chemistry (2022)
-
Ionophore constructed from non-covalent assembly of a G-quadruplex and liponucleoside transports K+-ion across biological membranes
Nature Communications (2020)