Abstract
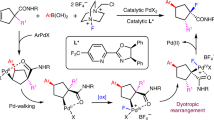
The direct and selective functionalization of C−H bonds of arenes is one of the most challenging yet valuable aims in organic synthesis. Despite notable recent achievements, a pre-installed directing group proved to be essential in most of the methodologies reported so far. In this context, the use of a transient directing group that can be generated in situ has attracted attention and demonstrated the great potential of this strategy. Here we report the use of an in situ generated palladacycle to accomplish remote-selective C−H alkylation reactions of arenes. Following the C−H functionalization event, the alkylated aryl ring undergoes a formal migration to provide diversely substituted benzofuran and indole scaffolds. Computational studies revealed that a palladium(IV) intermediate is not involved in the alkylation step. The aryl migration was found to proceed through a sequential C−C bond cleavage, insertion and β-hydride-elimination process. The increasing steric bulk that builds up during the C−H functionalization step drives the unusual C−C bond cleavage in a non-strained system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Astruc, D. Modern Arene Chemistry (Wiley-VCH, 2002).
Chen, X., Engle, K. M., Wang, D.-H. & Yu, J.-Q. Palladium(II)-catalyzed C–H activation/C−C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 48, 5094–5115 (2009).
Daugulis, O., Do, H.-Q. & Shabashov, D. Palladium- and copper-catalyzed arylation of carbon–hydrogen bonds. Acc. Chem. Res. 42, 1074–1086 (2009).
Colby, D. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 110, 624–655 (2010).
Engle, K. M., Mei, T.-S., Wasa, M. & Yu, J.-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 45, 788–802 (2012).
Neufeldt, S. R. & Sanford, M. S. Controlling site selectivity in palladium-catalyzed C–H bond functionalization. Acc. Chem. Res. 45, 936–946 (2012).
Huang, Z., Lim, H. N., Mo, F., Young, M. C. & Dong, G. Transition metal-catalyzed ketone-directed or mediated C–H functionalization. Chem. Soc. Rev. 44, 7764–7786 (2015).
Gensch, T., Hopkinson, M. N., Glorius, F. & Wencel-Delord, J. Mild metal-catalyzed C–H activation: examples and concepts. Chem. Soc. Rev. 45, 2900–2936 (2016).
Hartwig, J. F. Evolution of C−H bond functionalization from methane to methodology. J. Am. Chem. Soc. 138, 2–24 (2016).
Davies, H. M. L. & Morton, D. Recent advances in C−H functionalization. J. Org. Chem. 81, 343–350 (2016).
Cho, J.-Y., Tse, M. K., Holmes, D., Maleczka, R. E. Jr & Smith, M. R. III. Remarkably selective iridium catalysts for the elaboration of aromatic C–H bonds. Science 295, 305–308 (2002).
Hartwig, J. F. Borylation and silylation of C–H bonds: a platform for diverse C–H bond functionalizations. Acc. Chem. Res. 45, 864–873 (2012).
Robbins, D. W. & Hartwig, J. F. Sterically controlled alkylation of arenes through iridium-catalyzed C–H borylation. Angew. Chem. Int. Ed. 52, 933–937 (2013).
Chen, C. & Hartwig, J. F. Rhodium-catalyzed intermolecular C–H silylation of arenes with high steric regiocontrol. Science 343, 853–857 (2014).
Phipps, R. J. & Gaunt, M. J. A meta-selective copper-catalyzed C–H bond arylation. Science 323, 1593–1597 (2009).
Duong, H. A., Gilligan, R. E., Cooke, M. L., Phipps, R. J. & Gaunt, M. J. Copper(II)-catalyzed meta-selective direct arylation of α-aryl carbonyl compounds. Angew. Chem. Int. Ed. 50, 463–466 (2011).
Zhang, Y.-H., Shi, B.-F. & Yu, J.-Q. Pd(II)-catalyzed olefination of electron-deficient arenes using 2,6-dialkylpyridine ligands. J. Am. Chem. Soc. 131, 5072–5074 (2009).
Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature 486, 518–522 (2012).
Wan, L., Dastbaravardeh, N., Li, G. & Yu, J.-Q. Cross-coupling of remote meta-C–H bonds directed by a U-shaped template. J. Am. Chem. Soc. 135, 18056–18059 (2013).
Tang, R.-Y., Li, G. & Yu, J.-Q. Conformation-induced remote meta-C–H activation of amines. Nature 507, 215–220 (2014).
Yang, G.-Q. et al. Pd(II)-catalyzed meta-C–H olefination, arylation, and acetoxylation of indolines using a U-shaped template. J. Am. Chem. Soc. 136, 10807–10813 (2014).
Bag, S. et al. Remote para-C−H functionalization of arenes by a D-shaped biphenyl template-based assembly. J. Am. Chem. Soc. 137, 11888–11891 (2015).
Catellani, M., Frignani, F. & Rangoni, A. A complex catalytic cycle leading to a regioselective synthesis of o,o′-disubstituted vinylarenes. Angew. Chem. Int. Ed. Engl. 36, 119–122 (1997).
Della Ca’, N., Fontana, M., Motti, E. & Catellani, M. Pd/norbornene: a winning combination for selective aromatic functionalization via C−H bond activation. Acc. Chem. Res. 49, 1389–1400 (2016).
Ye, J. & Lautens, M. Palladium-catalysed norbornene-mediated C−H functionalization of arenes. Nat. Chem. 7, 863–870 (2015).
Wang, X.-C. et al. Ligand-enabled meta-C–H activation using a transient mediator. Nature 519, 334–338 (2015).
Shen, P.-X., Wang, X.-C., Wang, P., Zhu, R.-Y. & Yu, J.-Q. Ligand-enabled meta-C−H alkylation and arylation using a modified norbornene. J. Am. Chem. Soc. 137, 11574–11577 (2015).
Dong, Z., Wang, J. & Dong, G. Simple amine-directed meta-selective C–H arylation via Pd/norbornene catalysis. J. Am. Chem. Soc. 137, 5887–5890 (2015).
Bedford, R. B., Coles, S. J., Hursthouse, M. B. & Limmert, M. E. The catalytic intermolecular orthoarylation of phenols. Angew. Chem. Int. Ed. 42, 112–114 (2003).
Luo, J., Preciado, S. & Larrosa, I. Overriding ortho−para selectivity via a traceless directing group relay strategy: the meta-selective arylation of phenols. J. Am. Chem. Soc. 136, 4109–4112 (2014).
Mo, F. & Dong, G. Regioselective ketone α-alkylation with simple olefins via dual activation. Science 345, 68–72 (2014).
Xu, Y., Young, M. C., Wang, C., Magness, D. M. & Dong, G. Catalytic C(sp3)−H arylation of free primary amines with an exo directing group generated in situ. Angew. Chem. Int. Ed. 55, 9084–9087 (2016).
Zhang, F.-L., Hong, K., Li, T.-J., Park, H. & Yu, J.-Q. Functionalization of C(sp3)–H bonds using a transient directing group. Science 351, 252–256 (2016).
Saidi, O. et al. Ruthenium-catalyzed meta sulfonation of 2-phenylpyridines. J. Am. Chem. Soc. 133, 19298–19301 (2011).
Paterson, A. J., John-Campbell, S. S., Mahon, M. F., Press, N. J. & Frost, C. G. Catalytic meta-selective C–H functionalization to construct quaternary carbon centres. Chem. Commun. 51, 12807–12810 (2015).
Hofmann, N. & Ackermann, L. meta-Selective C−H bond alkylation with secondary alkyl halides. J. Am. Chem. Soc. 135, 5877–5884 (2013).
Li, J. et al. N-Acyl amino acid ligands for ruthenium(II)-catalyzed meta-C−H tert-alkylation with removable auxiliaries. J. Am. Chem. Soc. 137, 13894–13901 (2015).
Satoh, T. & Miura, M. Catalytic processes involving β-carbon elimination. Top. Organomet. Chem. 14, 1–20 (2005).
Dong, G. C–C Bond Activation (Topics in Current Chemistry 346, Springer, 2014).
Dermenci, A., Coe, J. W. & Dong, G. Direct activation of relatively unstrained carbon–carbon bonds in homogeneous systems. Org. Chem. Front. 1, 567–581 (2014).
Lu, Z. et al. Water-controlled regioselectivity of Pd-catalyzed domino reaction involving a C–H activation process: rapid synthesis of diverse carbo- and heterocyclic skeletons. Org. Lett. 12, 480–483 (2010).
Sickert, M., Weinstabl, H., Peters, B., Hou, X. & Lautens, M. Intermolecular domino reaction of two aryl iodides involving two C–H functionalizations. Angew. Chem. Int. Ed. 53, 5147–5151 (2014).
Huang, Q., Fazio, A., Dai, G., Campo, M. A. & Larock, R. C. Pd-catalyzed alkyl to aryl migration and cyclization: an efficient synthesis of fused polycycles via multiple C–H activation. J. Am. Chem. Soc. 126, 7460–7461 (2004).
Piou, T., Bunescu, A., Wang, Q., Neuville, L. & Zhu, J. Palladium-catalyzed through-space C(sp3)–H and C(sp2)–H bond activation by 1,4-palladium migration: efficient synthesis of [3,4]-fused oxindoles. Angew. Chem. Int. Ed. 52, 12385–12389 (2013).
Bunescu, A., Piou, T., Wang, Q. & Zhu, J. Pd-catalyzed dehydrogenative aryl−aryl bond formation via double C(sp2)−H bond activation: efficient synthesis of [3,4]-fused oxindoles. Org. Lett. 17, 334–337 (2015).
Grigg, R., Fretwell, P., Meerholtz, C. & Sridharan, V. Palladium catalysed synthesis of spiroindolines. Tetrahedron 50, 359–370 (1994).
Ruck, R. T. et al. Palladium-catalyzed tandem Heck reaction/C–H functionalization—preparation of spiro-indane-oxindoles. Angew. Chem. Int. Ed. 47, 4711–4714 (2008).
Piou, T., Neuville, L. & Zhu, J. Spirocyclization by palladium-catalyzed domino Heck–direct C–H arylation reactions: synthesis of spirodihydroquinolin-2-ones. Org. Lett. 14, 3760–3763 (2012).
Piou, T., Neuville, L. & Zhu, J. Activation of a C(sp3)–H bond by a transient σ-alkylpalladium(II) complex: synthesis of spirooxindoles through a palladium-catalyzed domino carbopalladation/C(sp3)–C(sp3) bond-forming process. Angew. Chem. Int. Ed. 51, 11561–11565 (2012).
Hopkinson, M. N., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Sperger, T., Sanhueza, I. A., Kalvet, I. & Schoenebeck, F. Computational studies of synthetically relevant homogeneous organometallic catalysis involving Ni, Pd, Ir, and Rh: an overview of commonly employed DFT methods and mechanistic insights. Chem. Rev. 115, 9532–9586 (2015).
Li, J. J. Heterocyclic Chemistry in Drug Discovery (John Wiley & Sons, 2013).
Acknowledgements
We are grateful for financial support from the Natural Sciences and Engineering Research Council (NSERC), the University of Toronto (U of T) and Alphora Research Inc. M.L. thanks the Canada Council for the Arts for a Killam Fellowship. A. Lough (U of T) is acknowledged for X-ray analysis. Z.S. is a visiting scholar from China Pharmaceutical University and thanks the China Scholarship Council. F.S. and T.S. thank the RWTH Aachen and the Evonik Foundation (scholarship to T.S.) for funding. Y.Y. thanks the Osaka University Scholarship for Overseas Research Activities 2015. C.K. is grateful for the award of a Synthesis and Solid State Pharmaceutical Centre PhD Scholarship. D. Schmidmeier is acknowledged for the synthesis of some substrates. We also thank T. Rovis (Columbia University) for suggestions on the mechanism of the reaction and providing chemicals and equipment during the revision of this manuscript, S. S. Stahl (University of Wisconsin-Madison), M. S. Taylor (U of T), D. A. Petrone (U of T), C. C. J. Loh (Max-Planck-Institut für Molekulare Physiologie, Abteilung Chemische Biologie) and I. A. Sanhueza (RWTH Aachen) for helpful discussions. M. Sickert (University of Leipzig) is acknowledged for his initial exploration in this area. This paper is dedicated to Professor Barry M. Trost on the occasion of his 75th birthday.
Author information
Authors and Affiliations
Contributions
J.Y. and M.L. conceived the idea. J.Y. and Z.S. conducted the majority of the experimental work and analysed data. T.S. and F.S. carried out the computational studies. Y.Y. and C.K. performed some preliminary screening reactions and substrate synthesis. J.Y., M.L., T.S. and F.S. prepared the manuscript with feedback from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 11448 kb)
Supplementary information
Crystallographic data for compound 2ca (CIF 3292 kb)
Supplementary information
Crystallographic data for compound 4b (CIF 945 kb)
Supplementary information
Crystallographic data for compound 4n (CIF 1397 kb)
Rights and permissions
About this article
Cite this article
Ye, J., Shi, Z., Sperger, T. et al. Remote C−H alkylation and C−C bond cleavage enabled by an in situ generated palladacycle. Nature Chem 9, 361–368 (2017). https://doi.org/10.1038/nchem.2631
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2631
This article is cited by
-
Nickel-catalyzed switchable arylative/endo-cyclization of 1,6-enynes
Nature Communications (2024)
-
Tuning hydrogenation chemistry of Pd-based heterogeneous catalysts by introducing homogeneous-like ligands
Nature Communications (2023)
-
Catalytic 4-exo-dig carbocyclization for the construction of furan-fused cyclobutanones and synthetic applications
Nature Communications (2023)
-
Reversible C–C bond formation using palladium catalysis
Nature Chemistry (2022)
-
Sila-spirocyclization involving unstrained C(sp3)−Si bond cleavage
Nature Communications (2022)