Abstract
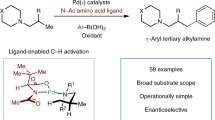
Transition-metal-catalysed direct C–H bond functionalization of aliphatic amines is of great importance in organic and medicinal chemistry research. Several methods have been developed for the direct sp3 C–H functionalization of secondary and tertiary aliphatic amines, but site-selective functionalization of primary aliphatic amines in remote positions remains a challenge. Here, we report the direct, highly site-selective γ-arylation of primary alkylamines via a palladium-catalysed C–H bond functionalization process on unactivated sp3 carbons. Using glyoxylic acid as an inexpensive, catalytic and transient directing group, a wide array of γ-arylated primary alkylamines were prepared without any protection or deprotection steps. This approach provides straightforward access to important structural motifs in organic and medicinal chemistry without the need for pre-functionalized substrates or stoichiometric directing groups and is demonstrated here in the synthesis of analogues of the immunomodulatory drug fingolimod directly from commercially available 2-amino-2-propylpropane-1,3-diol.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kittakoop, P., Mahidol, C. & Ruchirawat, S. Alkaloids as important scaffolds in therapeutic drugs for the treatments of cancer, tuberculosis, and smoking cessation. Curr. Top. Med. Chem. 14, 239–252 (2014).
Cushnie, T. P. T., Cushnie, B. & Lamb, A. J. Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 44, 377–386 (2014).
McGrath, N. A., Brichacek, M. & Njardarson, J. T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 87, 1348–1349 (2010).
Salvatore, R. N., Yoon, C. H. & Jung, K. W. Synthesis of secondary amines. Tetrahedron 57, 7785–7811 (2001).
Hager, A., Vrielink, N., Hager, D., Lefranc, J. & Trauner, D. Synthetic approaches towards alkaloids bearing α-tertiary amines. Nat. Prod. Rep. 33, 491–522 (2016).
Chen, X., Engle, K. M., Wang, D.-H. & Yu, J.-Q. Palladium(II)-catalyzed C–H activation/C–C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 48, 5094–5115 (2009).
Baudoin, O. Transition metal-catalyzed arylation of unactivated C(sp3)–H bonds. Chem. Soc. Rev. 40, 4902–4911 (2011).
Li, H., Li, B.-J. & Shi, Z.-J. Challenge and progress: palladium-catalyzed sp3 C–H activation. Catal. Sci. Technol. 1, 191–206 (2011).
White, M. C. Adding aliphatic C–H bond oxidations to synthesis. Science 335, 807–809 (2012).
Yamaguchi, J., Yamaguchi, A. D. & Itami, K. C–H bond functionalization emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012).
Colby, D. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 110, 624–655 (2010).
Daugulis, O., Roane, J. & Tran, L. D. Bidentate, monoanionic auxiliary-directed functionalization of carbon–hydrogen bonds. Acc. Chem. Res. 48, 1053–1064 (2015).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Rouquet, G. & Chatani, N. Catalytic functionalization of C(sp2)–H and C(sp3)–H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 52, 11726–11743 (2013).
Chen, Z. et al. Transition metal-catalyzed C–H bond functionalizations by the use of diverse directing groups. Org. Chem. Front. 2, 1107–1295 (2015).
He, G., Wang, B., Nack, W. A. & Chen, G. Syntheses and transformations of α-amino acids via palladium-catalyzed auxiliary-directed sp3 C–H functionalization. Acc. Chem. Res. 49, 635–645 (2016).
Yoo, W.-J. & Li, C.-J. Cross-dehydrogenative coupling reactions of sp3-hybridized C–H bonds. Top. Curr. Chem. 292, 281–302 (2010).
Yeung, C. S. & Dong, V. M. Catalytic dehydrogenative cross-coupling: forming carbon–carbon bonds by oxidizing two carbon–hydrogen bonds. Chem. Rev. 111, 1215–1292 (2011).
Zhang, C., Tang, C. & Jiao, N. Recent advances in copper-catalyzed dehydrogenative functionalization via single electron transfer (SET) process. Chem. Soc. Rev. 41, 3464–3484 (2012).
Girard, S. A., Knauber, T. & Li, C.-J. The cross-dehydrogenative coupling of Csp3–H bonds: a versatile strategy for C–C bond formations. Angew. Chem. Int. Ed. 53, 74–100 (2014).
Spangler, J. E., Kobayashi, Y., Verma, P., Wang, D.-H. & Yu, J.-Q. α-Arylation of saturated azacycles and N-methylamines via palladium(II)-catalyzed C(sp3)–H coupling. J. Am. Chem. Soc. 137, 11876–11879 (2015).
Pastine, S. J., Gribkov, D. V. & Sames, D. sp3 C–H bond arylation directed by amidine protecting group: α-arylation of pyrrolidines and piperidines. J. Am. Chem. Soc. 128, 14220–14221 (2006).
Chatani, N. et al. Carbonylation at sp3 C–H bonds adjacent to a nitrogen atom in alkylamines catalyzed by rhodium complexes. J. Am. Chem. Soc. 122, 12882–12883 (2000).
McNally, A., Haffemayer, B., Collins, B. S. L. & Gaunt, M. J. Palladium-catalysed C–H activation of aliphatic amines to give strained nitrogen heterocycles. Nature 510, 129–133 (2014).
Topczewski, J. J., Cabrera, P. J., Saper, N. I. & Sanford, M. S. Palladium-catalysed transannular C–H functionalization of alicyclic amines. Nature 531, 220–224 (2016).
Zaitsev, V. G., Shabashov, D. & Daugulis, O. Highly regioselective arylation of sp3 C−H bonds catalyzed by palladium acetate. J. Am. Chem. Soc. 127, 13154–13155 (2005).
He, G., Zhao, Y., Zhang, S., Lu, C. & Chen, G. Highly efficient syntheses of azetidines, pyrrolidines and indolines via palladium-catalyzed intramolecular amination of C(sp3) and C(sp2)–H bonds at γ and δ positions. J. Am. Chem. Soc. 134, 3–6 (2012).
Zhang, S.-Y. et al. Efficient alkyl ether synthesis via palladium-catalyzed, picolinamide-directed alkoxylation of unactivated C(sp3)–H and C(sp2)–H bonds at remote positions. J. Am. Chem. Soc. 134, 7313–7316 (2012).
Zhang, S.-Y. et al. Palladium-catalyzed picolinamide-directed alkylation of unactivated C(sp3)–H bonds with alkyl iodides. J. Am. Chem. Soc. 135, 2124–2127 (2013).
Rodríguez, N., Romero-Revilla, J. A., Fernández-Ibáñez, M. Á. & Carretero, J. C. Palladium-catalyzed N-(2-pyridyl)sulfonyl-directed C(sp3)–H γ-arylation of amino acid derivatives. Chem. Sci. 4, 175–179 (2013).
Chan, K. S. L. et al. Ligand-enabled cross-coupling of C(sp3)–H bonds with arylboron reagents via Pd(II)/Pd(0) catalysis. Nat. Chem. 6, 146–150 (2014).
Calleja, J. et al. A steric tethering approach enables palladium-catalysed C–H activation of primary amino alcohols. Nat. Chem. 7, 1009–1016 (2015).
Huang, Z., Wang, C. & Dong, G. A hydrazone-based exo-directing group strategy for β-C–H oxidation of aliphatic amines. Angew. Chem. Int. Ed. 55, 5299–5303 (2016).
Jun, C.-H., Lee, H. & Hong, J.-B. Chelation-assisted intermolecular hydroacylation: direct synthesis of ketone from aldehyde and 1-alkene. J. Org. Chem. 62, 1200–1201 (1997).
Rousseau, G. & Breit, B. Removable directing groups in organic synthesis and catalysis. Angew. Chem. Int. Ed. 50, 2450–2494 (2011).
Dydio, P. & Reek, J. N. H. Supramolecular control of selectivity in transition-metal catalysis through substrate preorganization. Chem. Sci. 5, 2135–2145 (2014).
Mo, F. & Dong, G. Regioselective ketone α-alkylation with simple olefins via dual activation. Science 345, 68–72 (2014).
Zhang, F.-L., Hong, K., Li, T.-J., Park, H. & Yu, J.-Q. Functionalization of C(sp3)–H bonds using a transient directing group. Science 351, 252–256 (2016).
Lapointe, D. & Fagnou, K. Overview of the mechanistic work on the concerted metallationdeprotonation pathway. Chem. Lett. 39, 1118–1126 (2010).
Arroniz, C., Denis, J. G., Ironmonger, A., Rassias, G. & Larrosa, I. An organic cation as a silver(I) analogue for the arylation of sp2 and sp3 C–H bonds with iodoarenes. Chem. Sci. 5, 3509–3514 (2014).
Weibel, J.-M., Blanc, A. & Pale, P. Ag-mediated reactions: coupling and heterocyclization reactions. Chem. Rev. 108, 3149–3173 (2008).
Xu, Y., Young, M. C., Wang, C., Magness, D. M. & Dong, G. Catalytic C(sp3)–H arylation of free primary amines with an exo directing group generated in situ. Angew. Chem. Int. Ed. 55, 9084–9087 (2016).
Acknowledgements
The authors acknowledge Indiana University–Purdue University Indianapolis and NSF CHE-1350541 for financial support. The authors thank J. Miao and K. Yang for discussions on this project.
Author information
Authors and Affiliations
Contributions
Y.L. and H.G. conceived and designed the experiments. Y.L. performed the experiments. Y.L. and H.G. analysed the data. H.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4271 kb)
Supplementary information
Crystallographic data for compound 6. (CIF 687 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Ge, H. Site-selective C–H arylation of primary aliphatic amines enabled by a catalytic transient directing group. Nature Chem 9, 26–32 (2017). https://doi.org/10.1038/nchem.2606
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2606
This article is cited by
-
Modeling the alkylation of amines with alkyl bromides: explaining the low selectivity due to multiple alkylation
Journal of Molecular Modeling (2024)
-
Transient directing ligands for selective metal-catalysed C–H activation
Nature Reviews Chemistry (2021)
-
Nonconventional driving force for selective oxidative C–C coupling reaction due to concurrent and curious formation of Ag0
Scientific Reports (2021)
-
Imine as a linchpin approach for meta-C–H functionalization
Nature Communications (2021)
-
Nickel-catalyzed deaminative Sonogashira coupling of alkylpyridinium salts enabled by NN2 pincer ligand
Nature Communications (2021)