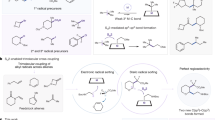
Abstract
Efficient C–H functionalization requires selectivity for specific C–H bonds. Progress has been made for directed aromatic substitution reactions to achieve ortho and meta selectivity, but a general strategy for para-selective C–H functionalization has remained elusive. Herein we introduce a previously unappreciated concept that enables nearly complete para selectivity. We propose that radicals with high electron affinity elicit arene-to-radical charge transfer in the transition state of radical addition, which is the factor primarily responsible for high positional selectivity. We demonstrate with a simple theoretical tool that the selectivity is predictable and show the utility of the concept through a direct synthesis of aryl piperazines. Our results contradict the notion, widely held by organic chemists, that radical aromatic substitution reactions are inherently unselective. The concept of radical substitution directed by charge transfer could serve as the basis for the development of new, highly selective C–H functionalization reactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Taylor, R. Electrophilic Aromatic Substitution (John Wiley & Sons, 1990).
Wencel-Delord, J. & Glorius, F. C–H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nature Chem. 5, 369–375 (2013).
Kuhl, N., Hopkinson, M. N., Wencel-Delord, J. & Glorius, F. Beyond directing groups: transition-metal-catalyzed C–H activation of simple arenes. Angew. Chem. Int. Ed. 51, 10236–10254 (2012).
Engle, K. M., Mei, T.-S., Wasa, M. & Yu, J.-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 45, 788–802 (2011).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C−H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature 486, 518–522 (2012).
Cheng, C. & Hartwig, J. F. Rhodium-catalyzed intermolecular C–H silylation of arenes with high steric regiocontrol. Science 343, 853–857 (2014).
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C−H activation for the construction of C−B bonds. Chem. Rev. 110, 890–931 (2009).
Saito, Y., Segawa, Y. & Itami, K. para-C–H borylation of benzene derivatives by a bulky iridium catalyst. J. Am. Chem. Soc. 137, 5193–5198 (2015).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C–H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Sibbald, P. A., Rosewall, C. F., Swartz, R. D. & Michael, F. E. Mechanism of N-fluorobenzenesulfonimide promoted diamination and carboamination reactions: divergent reactivity of a Pd(IV) species. J. Am. Chem. Soc. 131, 15945–15951 (2009).
Wang, X., Leow, D. & Yu, J.-Q. Pd(II)-catalyzed para-selective C–H arylation of monosubstituted arenes. J. Am. Chem. Soc. 133, 13864–13867 (2011).
Carey, F. A. & Sundberg, R. J. Advanced Organic Chemistry B: Reaction and Synthesis 1052–1053 (Springer, 2007).
Boursalian, G. B., Ngai, M.-Y., Hojczyk, K. N. & Ritter, T. Pd-catalyzed aryl C–H imidation with arene as the limiting reagent. J. Am. Chem. Soc. 135, 13278–13281 (2013).
Fischer, H. & Radom, L. Factors controlling the addition of carbon-centered radicals to alkenes—an experimental and theoretical perspective. Angew. Chem. Int. Ed. 40, 1340–1371 (2001).
Wong, M. W., Pross, A. & Radom, L. Comparison of the addition of CH3•, CH2OH•, and CH2CN• radicals to substituted alkenes: a theoretical study of the reaction mechanism. J. Am. Chem. Soc. 116, 6284–6292 (1994).
Wong, M. W., Pross, A. & Radom, L. Addition of tert-butyl radical to substituted alkenes: a theoretical study of the reaction mechanism. J. Am. Chem. Soc. 116, 11938–11943 (1994).
Parr, R. G. & Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 106, 4049–4050 (1984).
Lewars, E. Computational Chemistry 425–436 (Kluwer Academic, 2003).
Ayers, P. W. & Levy, M. Perspective on ‘Density functional approach to the frontier-electron theory of chemical reactivity’. Theor. Chem. Acc. 103, 353–360 (2000).
Traynham, J. G. Ipso substitution in free-radical aromatic substitution reactions. Chem. Rev. 79, 323–330 (1979).
Bloom, S. et al. A polycomponent metal-catalyzed aliphatic, allylic, and benzylic fluorination. Angew. Chem. Int. Ed. 51, 10580–10583 (2012).
Pitts, C. R. et al. Direct, catalytic monofluorination of sp3 C–H bonds: a radical-based mechanism with ionic selectivity. J. Am. Chem. Soc. 136, 9780–9791 (2014).
Michaudel, Q., Thevenet, D. & Baran, P. S. Intermolecular Ritter-type C–H amination of unactivated sp3 carbons. J. Am. Chem. Soc. 134, 2547–2550 (2012).
Cheng, Y., Gu, X. & Li, P. Visible-light photoredox in homolytic aromatic substitution: direct arylation of arenes with aryl halides. Org. Lett. 15, 2664–2667 (2013).
Allen, L. J., Cabrera, P. J., Lee, M. & Sanford, M. S. N-Acyloxyphthalimides as nitrogen radical precursors in the visible light photocatalyzed room temperature C–H amination of arenes and heteroarenes. J. Am. Chem. Soc. 136, 5607–5610 (2014).
Citterio, A. et al. Polar effects in free radical reactions. Homolytic aromatic amination by the amino radical cation, •+NH3: reactivity and selectivity. J. Org. Chem. 49, 4479–4482 (1984).
Minisci, F. Novel applications of free-radical reactions in preparative organic chemistry. Synthesis 1–24 (1973).
Minisci, F. Nuovi processi per introdurre l'azoto in molecole organiche con reazioni radicaliche: azidazione e amminazione. Chim. Ind. (Milano) 49, 705–719 (1967).
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Fischer, C. & Koenig, B. Palladium- and copper-mediated N-aryl bond formation reactions for the synthesis of biological active compounds. Beilstein J. Org. Chem. 7, 59–74 (2011).
Acknowledgements
The authors thank the National Institute of General Medical Sciences (GM088237), the National Institute of Biomedical Imaging and Bioengineering (EB013042), UCB Pharma and Kwanjeong Educational Foundation for funding. The authors further thank J. McClean (Harvard University) for helpful discussions.
Author information
Authors and Affiliations
Contributions
G.B.B., W.S.H. and A.R.M. designed and performed the experiments and analysed the data. G.B.B. discovered the Ar–TEDA formation reaction and conceived the mechanistic proposal and explanation for the positional selectivity. A.R.M. discovered the reduction of Ar–TEDA compounds to aryl piperazines. G.B.B. and T.R. prepared the manuscript with input from W.S.H. and A.R.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5443 kb)
Rights and permissions
About this article
Cite this article
Boursalian, G., Ham, W., Mazzotti, A. et al. Charge-transfer-directed radical substitution enables para-selective C–H functionalization. Nature Chem 8, 810–815 (2016). https://doi.org/10.1038/nchem.2529
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2529
This article is cited by
-
C–H Bond Functionalization of N-Heteroarenes Mediated by Selectfluor
Topics in Current Chemistry (2023)
-
The interplay of polar effects in controlling the selectivity of radical reactions
Nature Synthesis (2022)
-
Organocatalyst-controlled site-selective arene C–H functionalization
Nature Chemistry (2021)
-
Late-stage C–H functionalization offers new opportunities in drug discovery
Nature Reviews Chemistry (2021)
-
A photochemical dehydrogenative strategy for aniline synthesis
Nature (2020)