Abstract
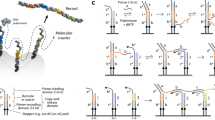
Modern-day factory assembly lines often feature robots that pick up, reposition and connect components in a programmed manner. The idea of manipulating molecular fragments in a similar way has to date only been explored using biological building blocks (specifically DNA). Here, we report on a wholly artificial small-molecule robotic arm capable of selectively transporting a molecular cargo in either direction between two spatially distinct, chemically similar, sites on a molecular platform. The arm picks up/releases a 3-mercaptopropanehydrazide cargo by formation/breakage of a disulfide bond, while dynamic hydrazone chemistry controls the cargo binding to the platform. Transport is controlled by selectively inducing conformational and configurational changes within an embedded hydrazone rotary switch that steers the robotic arm. In a three-stage operation, 79–85% of 3-mercaptopropanehydrazide molecules are transported in either (chosen) direction between the two platform sites, without the cargo at any time fully dissociating from the machine nor exchanging with other molecules in the bulk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Feynman, R. P. There's plenty of room at the bottom. Eng. Sci. 23, 22–36 (1960).
Drexler, K. E. Molecular engineering: an approach to the development of general capabilities for molecular manipulation. Proc. Natl Acad. Sci. USA 78, 5275–5278 (1981).
Drexler, K. E. Nanosystems: Molecular Machinery, Manufacturing, and Computation (Wiley, 1992).
Kumagai, T. et al. Controlling intramolecular hydrogen transfer in a porphycene molecule with single atoms or molecules located nearby. Nature Chem. 6, 41–46 (2014).
Smalley, R. E. Of chemistry, love and nanobots. Sci. Am. 285, 76–77 (2001).
Whitesides, G. M. The once and future nanomachine. Sci. Am. 285, 78–83 (2001).
Jones, R. A. L. Soft Machines: Nanotechnology and Life (Oxford Univ. Press, 2004).
Maier, T., Leibundgut, M. & Ban, N. The crystal structure of a mammalian fatty acid synthase. Science 321, 1315–1322 (2008).
Brignole, E. J., Smith, S. & Asturias, F. J. Conformational flexibility of metazoan fatty acid synthase enables catalysis. Nature Struct. Mol. Biol. 16, 190–197 (2009).
Chan, D. I. & Vogel, H. J. Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem. J. 430, 1–19 (2010).
Ding, B. & Seeman, N. C. Operation of a DNA robot arm inserted into a 2D DNA crystalline substrate. Science 314, 1583–1585 (2006).
Gu, H., Chao, J., Xiao, S.-J. & Seeman, N. C. A proximity-based programmable DNA nanoscale assembly line. Nature 465, 202–205 (2010).
von Delius, M. & Leigh, D. A. Walking molecules. Chem. Soc. Rev. 40, 3656–3676 (2011).
Berná, J. et al. Macroscopic transport by synthetic molecular machines. Nature Mater. 4, 704–710 (2005).
Liu, Y. et al. Linear artificial molecular muscles. J. Am. Chem. Soc. 127, 9745–9759 (2005).
Eelkema, R. et al. Molecular machines: nanomotor rotates microscale objects. Nature 440, 163 (2006).
Li, Q. et al. Macroscopic contraction of a gel induced by the integrated motion of light-driven molecular motors. Nature Nanotech. 10, 161–165 (2015).
Kay, E. R. & Leigh, D. A. Rise of the molecular machines. Angew. Chem. Int. Ed. 54, 10080–10088 (2015).
Muraoka, T., Kinbara, K. & Aida, T. Mechanical twisting of a guest by a photoresponsive host. Nature 440, 512–515 (2006).
Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T. & Nussbaumer, A. L. Artificial molecular machines. Chem. Rev. 115, 10081–10206 (2015).
Astumian, R. D. Design principles for Brownian molecular machines: how to swim in molasses and walk in a hurricane. Phys. Chem. Chem. Phys. 9, 5067–5083 (2007).
von Delius, M., Geertsema, E. M. & Leigh, D. A. A synthetic small molecule that can walk down a track. Nature Chem. 2, 96–101 (2010).
von Delius, M., Geertsema, E. M., Leigh, D. A. & Tang, D.-T. D. Design, synthesis, and operation of small molecules that walk along tracks. J. Am. Chem. Soc. 132, 16134–16145 (2010).
Barrell, M. J., Campaña, A. G., von Delius, M., Geertsema, E. M. & Leigh, D. A. Light-driven transport of a molecular walker in either direction along a molecular track. Angew. Chem. Int. Ed. 50, 285–290 (2011).
Feringa, B. L. & Browne, W. R. (eds) Molecular Switches 2nd edn (Wiley-VCH, 2011).
Su, X. & Aprahamian, I. Switching around two axles: controlling the configuration and conformation of a hydrazone-based switch. Org. Lett. 13, 30–33 (2011).
Su, X., Robbins, T. F. & Aprahamian, I. Switching through coordination-coupled proton transfer. Angew. Chem. Int. Ed. 50, 1841–1844 (2011).
Ray, D., Foy, J. T., Hughes, R. P. & Aprahamian, I. A switching cascade of hydrazone-based rotary switches through coordination-coupled proton relays. Nature Chem. 4, 757–762 (2012).
Japp, F. R. & Klingemann, F. Ueber benzolazo- und benzolhydrazofettsäuren. Ber. Dtsch. Chem. Ges. 20, 2942–2944 (1887).
Landge, S. L. et al. Isomerization mechanism in hydrazone-based rotary switches: lateral shift, rotation, or tautomerization? J. Am. Chem. Soc. 133, 9812–9823 (2011).
Astumian, R. D. & Derényi, I. Fluctuation driven transport and models of molecular motors and pumps. Eur. Biophys. J. 27, 474–489 (1998).
Hernández, J. V., Kay, E. R. & Leigh, D. A. A reversible synthetic rotary molecular motor. Science 306, 1532–1537 (2004).
Ragazzon, G., Baroncini, M., Silvi, S., Venturi, M. & Credi, A. Light-powered autonomous and directional molecular motion of a dissipative self-assembling system. Nature Nanotech. 10, 70–75 (2015).
Lewandowski, B. et al. Sequence-specific peptide synthesis by an artificial small-molecule machine. Science 339, 189–193 (2013).
Acknowledgements
The authors acknowledge the Engineering and Physical Sciences Research Council (EPSRC) for funding and the EPSRC National Mass Spectrometry Service Centre (Swansea, UK) for high-resolution mass spectrometry.
Author information
Authors and Affiliations
Contributions
A.M. and D.A.L. planned the project. S.K., A.T.L.L., A.M. and J.S. carried out the experimental work. D.A.L. directed the research. All authors contributed to the analysis of the results and the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 7758 kb)
Rights and permissions
About this article
Cite this article
Kassem, S., Lee, A., Leigh, D. et al. Pick-up, transport and release of a molecular cargo using a small-molecule robotic arm. Nature Chem 8, 138–143 (2016). https://doi.org/10.1038/nchem.2410
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2410
This article is cited by
-
Ratcheting synthesis
Nature Reviews Chemistry (2023)
-
Using supramolecular machinery to engineer directional charge propagation in photoelectrochemical devices
Nature Chemistry (2023)
-
Functionalization of BODIPY Dyes with Additional C–N Double Bonds and Their Applications
Topics in Current Chemistry (2023)
-
Pumping between phases with a pulsed-fuel molecular ratchet
Nature Nanotechnology (2022)
-
Moving forward in the semantic soup of artificial molecular machine taxonomy
Nature Nanotechnology (2022)