Abstract
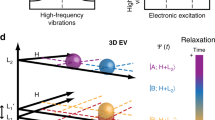
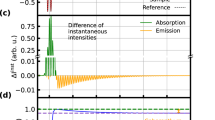
Determining the initial pathway for ultrafast energy redistribution within biomolecules is a challenge, and haem proteins, for which energy can be deposited locally in the haem moiety using short light pulses, are suitable model systems to address this issue. However, data acquired using existing experimental techniques that fail to combine sufficient structural sensitivity with adequate time resolution have resulted in alternative hypotheses concerning the interplay between energy flow among highly excited vibrational levels and potential concomitant electronic processes. By developing a femtosecond-stimulated Raman set-up, endowed with the necessary tunability to take advantage of different resonance conditions, here we visualize the temporal evolution of energy redistribution over different vibrational modes in myoglobin. We establish that the vibrational energy initially stored in the highly excited Franck–Condon manifold is transferred with different timescales into low- and high-frequency modes, prior to slow dissipation through the protein. These findings demonstrate that a newly proposed mechanism involving the population dynamics of specific vibrational modes settles the controversy on the existence of transient electronic intermediates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Leitner, D. M. Energy flow in proteins. Annu. Rev. Phys. Chem. 59, 233–259 (2008).
Mizutani, Y. & Kitagawa, T. Direct observation of cooling of heme upon photodissociation of carbonmonoxy myoglobin. Science 278, 443–446 (1997).
Miller, R. J. D. Vibrational energy relaxation and structural dynamics of heme proteins. Annu. Rev. Phys. Chem. 42, 581–614 (1991).
Henry, E. R., Eaton, W. A. & Hochstrasser, R. M. Molecular dynamics simulations of cooling in laser-excited heme proteins. Proc. Natl Acad. Sci. USA 83, 8982–8986 (1986).
Mukamel, S. Principles of Nonlinear Optical Spectroscopy (Oxford Univ. Press, 1995).
Brunori, M. Myoglobin strikes back. Protein Sci. 19, 195–201 (2010).
Lim, M., Jackson, T. A. & Anfinrud, P. A. Femtosecond near-IR absorbance study of photoexcited myoglobin: dynamics of electronic and thermal relaxation. J. Phys. Chem. 100, 12043–12051 (1996).
Ye, X. et al. Investigations of heme protein absorption line shapes, vibrational relaxation, and resonance Raman scattering on ultrafast time scales. J. Phys. Chem. A 107, 8156–8165 (2003).
Ye, X., Demidov, A. & Champion, P. M. Measurements of the photodissociation quantum yields of MbNO and MbO2 and the vibrational relaxation of the six-coordinate heme species. J. Am. Chem. Soc. 124, 5914–5924 (2002).
Kholodenko, Y., Volk, M., Gooding, E. & Hochstrasser, R. Energy dissipation and relaxation processes in deoxy myoglobin after photoexcitation in the Soret region. Chem. Phys. 259, 71–87 (2000).
Armstrong, M. R., Ogilvie, J. P., Cowan, M. L., Nagy, A. M. & Miller, R. J. D. Observation of the cascaded atomic-to-global length scales driving protein motion. Proc. Natl Acad. Sci. USA 100, 4990–4994 (2003).
Groot, M.-L. et al. Coherent infrared emission from myoglobin crystals: an electric field measurement. Proc. Natl Acad. Sci. USA 99, 1323–1328 (2002).
Franzen, S., Bohn, B., Poyart, C. & Martin, J. L. Evidence for sub-picosecond heme doming in hemoglobin and myoglobin: a time-resolved resonance Raman comparison of carbonmonoxy and deoxy species. Biochemistry 34, 1224–1237 (1995).
Petrich, J. W., Martin, J. L., Houde, D., Poyart, C. & Orszag, A. Time-resolved Raman spectroscopy with subpicosecond resolution: vibrational cooling and delocalization of strain energy in photodissociated (carbonmonoxy)hemoglobin. Biochemistry 26, 7914–7923 (1987).
Kruglik, S. G., Lambry, J.-C., Martin, J.-L., Vos, M. H. & Negrerie, M. Sub-picosecond Raman spectrometer for time-resolved studies of structural dynamics in heme proteins. J. Raman Spectrosc. 42, 265–275 (2011).
Simpson, M. C. et al. Transient Raman observations of heme electronic and vibrational photodynamics in deoxyhemoglobin. J. Am. Chem. Soc. 119, 5110–5117 (1997).
Li, P., Sage, J. T. & Champion, P. M. Probing picosecond processes with nanosecond lasers: electronic and vibrational relaxation dynamics of heme proteins. J. Chem. Phys. 97, 3214–3227 (1992).
Li, X. Y., Czernuszewicz, R. S., Kincaid, J. R., Stein, P. & Spiro, T. G. Consistent porphyrin force field. 2. Nickel octaethylporphyrin skeletal and substituent mode assignments from nitrogen-15, meso-d4, and methylene-d16 Raman and infrared isotope shifts. J. Phys. Chem. 94, 47–61 (1990).
Cornelius, P. A., Steele, A. W., Chernoff, D. A. & Hochstrasser, R. M. Different dissociation pathways and observation of an excited deoxy state in picosecond photolysis of oxy- and carboxymyoglobin. Proc. Natl Acad. Sci. USA 78, 7526–7529 (1981).
Lim, M., Jackson, T. & Anfinrud, P. Binding of CO to myoglobin from a heme pocket docking site to form nearly linear Fe–C–O. Science 269, 962–966 (1995).
Petrich, J. W., Poyart, C. & Martin, J. L. Photophysics and reactivity of heme proteins: a femtosecond absorption study of hemoglobin, myoglobin, and protoheme. Biochemistry 27, 4049–4060 (1988).
Schneebeck, M., Vigil, L. & Ondrias, M. Mode-selective energy localization during photoexcitation of deoxyhemoglobin and heme model complexes. Chem. Phys. Lett. 215, 251–256 (1993).
Franzen, S., Kiger, L., Poyart, C. & Martin, J.-L. Heme photolysis occurs by ultrafast excited state metal-to-ring charge transfer. Biophys. J. 80, 2372–2385 (2001).
Consani, C., Auböck, G., Bräm, O., van Mourik, F. & Chergui, M. A cascade through spin states in the ultrafast haem relaxation of met-myoglobin. J. Chem. Phys. 140, 025103 (2014).
Levantino, M. et al. Ultrafast myoglobin structural dynamics observed with an X-ray free-electron laser. Nature Commun. 6, 6772 (2015).
Kukura, P., McCamant, D. W. & Mathies, R. A. Femtosecond stimulated Raman spectroscopy. Annu. Rev. Phys. Chem. 58, 461–488 (2007).
Champion, P. M. Following the flow of energy in biomolecules. Science 310, 980–982 (2005).
Mukamel, S. & Biggs, J. D. Communication: comment on the effective temporal and spectral resolution of impulsive stimulated Raman signals. J. Chem. Phys. 134, 161101 (2011).
Fumero, G., Batignani, G., Dorfman, K. E., Mukamel, S. & Scopigno, T. On the resolution limit of femtosecond stimulated Raman spectroscopy: modelling fifth-order signals with overlapping pulses. ChemPhysChem 16, 3438–3443 (2015).
Sagnella, D. E., Straub, J. E., Jackson, T. A., Lim, M. & Anfinrud, P. A. Vibrational population relaxation of carbon monoxide in the heme pocket of photolyzed carbonmonoxy myoglobin: comparison of time-resolved mid-IR absorbance experiments and molecular dynamics simulations. Proc. Natl Acad. Sci. USA 96, 14324–14329 (1999).
Batignani, G. et al. Electronic resonances in broadband stimulated Raman spectroscopy. Sci. Rep. 6, 18445 (2016).
Stallard, B. R., Champion, P. M., Callis, P. R. & Albrecht, A. C. Advances in calculating Raman excitation profiles by means of the transform theory. J. Chem. Phys. 78, 712–722 (1983).
Siebrand, W. Radiationless transitions in polyatomic molecules. II. Triplet-ground-state transitions in aromatic hydrocarbons. J. Chem. Phys. 47, 2411–2422 (1967).
Englman, R. & Jortner, J. The energy gap law for radiationless transitions in large molecules. Mol. Phys. 18, 145–164 (1970).
Rosca, F. et al. Investigations of anharmonic low-frequency oscillations in heme proteins. J. Phys. Chem. A 106, 3540–3552 (2002).
Harbola, U., Umapathy, S. & Mukamel, S. Loss and gain signals in broadband stimulated-Raman spectra: theoretical analysis. Phys. Rev. A 88, 011801 (2013).
Loparo, J. J., Cheatum, C. M., Ondrias, M. R. & Simpson, M. Transient Raman observations of heme vibrational dynamics in five-coordinate iron porphyrins. Chem. Phys. 286, 353–374 (2003).
Leitner, D. M. Frequency-resolved communication maps for proteins and other nanoscale materials. J. Chem. Phys. 130, 195101 (2009).
Yoshizawa, M., Hattori, Y. & Kobayashi, T. Femtosecond time-resolved resonance Raman gain spectroscopy in polydiacetylene. Phys. Rev. B 49, 13259–13262 (1994).
McCamant, D. W., Kukura, P., Yoon, S. & Mathies, R. A. Femtosecond broadband stimulated Raman spectroscopy: apparatus and methods. Rev. Sci. Instrum. 75, 4971–4980 (2004).
Kukura, P., McCamant, D. W., Yoon, S., Wandschneider, D. B. & Mathies, R. A. Structural observation of the primary isomerization in vision with femtosecond-stimulated Raman. Science 310, 1006–1009 (2005).
Dietze, D. R. & Mathies, R. A. Femtosecond stimulated Raman spectroscopy. ChemPhysChem 17, 1224–1251 (2016).
Batignani, G. et al. Probing ultrafast photo-induced dynamics of the exchange energy in a Heisenberg antiferromagnet. Nature Photon. 9, 506–510 (2015).
Pontecorvo, E. et al. Femtosecond stimulated Raman spectrometer in the 320–520 nm range. Opt. Express 19, 1107–1112 (2011).
Pontecorvo, E., Ferrante, C., Elles, C. G. & Scopigno, T. Spectrally tailored narrowband pulses for femtosecond stimulated Raman spectroscopy in the range 330–750 nm. Opt. Express 21, 6866–6872 (2013).
Weigel, A. & Ernsting, N. Excited stilbene: intramolecular vibrational redistribution and solvation studied by femtosecond stimulated Raman spectroscopy. J. Phys. Chem. B 114, 7879–7893 (2010).
Pontecorvo, E., Ferrante, C., Elles, C. G. & Scopigno, T. Structural rearrangement accompanying the ultrafast electrocyclization reaction of a photochromic molecular switch. J. Phys. Chem. B 118, 6915–6921 (2014).
Laimgruber, S., Schachenmayr, H., Schmidt, B., Zinth, W. & Gilch, P. A femtosecond stimulated Raman spectrograph for the near ultraviolet. Appl. Phys. B 85, 557–564 (2006).
Kloz, M., van Grondelle, R. & Kennis, J. T. Correction for the time dependent inner filter effect caused by transient absorption in femtosecond stimulated Raman experiment. Chem. Phys. Lett. 544, 94–101 (2012).
Weigel, A. et al. Femtosecond stimulated Raman spectroscopy of flavin after optical excitation. J. Phys. Chem. B 115, 3656–3680 (2011).
Kruglik, S. G. et al. Picosecond primary structural transition of the heme is retarded after nitric oxide binding to heme proteins. Proc. Natl Acad. Sci. USA 107, 13678–13683 (2010).
Acknowledgements
T.S. thanks A. Arcovito for early discussions on the potential impact of an FSRS experiment in Mb, and acknowledges an inspiring visit to the Mathies lab. M. Aschi, G. Batignani, P. Champion, M. Garavelli, P. Kukura, Y. Mizutani, S. Kruglik and S. Mukamel provided invaluable input to this work. The authors thank B. Vallone and the Dipartimento di Scienze Biochimiche and Istituto Pasteur-Fondazione Cenci Bolognetti of Università di Roma La Sapienza for support with the sample preparation. T.S. is especially grateful to M. Brunori for continued support and critical insights. This research has received funding from the European Research Council under the European Union Seventh Framework Program (FP7/2007–2013) and no. 207916 (FEMTOSCOPY).
Author information
Authors and Affiliations
Contributions
T.S. directed the research. E.P. led the experimental activity with the assistance of C.F. and support from G.C. and T.S. C.F. performed data analysis and numerical modelling with the assistance of E.P. T.S. wrote the manuscript with C.F. and M.H.V. All the authors discussed the results and implications and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1191 kb)
Rights and permissions
About this article
Cite this article
Ferrante, C., Pontecorvo, E., Cerullo, G. et al. Direct observation of subpicosecond vibrational dynamics in photoexcited myoglobin. Nature Chem 8, 1137–1143 (2016). https://doi.org/10.1038/nchem.2569
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2569
This article is cited by
-
Carotenoid responds to excess energy dissipation in the LH2 complex from Rhodoblastus acidophilus
Photosynthesis Research (2022)
-
Improved spectral resolution of the femtosecond stimulated Raman spectroscopy achieved by the use of the 2nd-order diffraction method
Scientific Reports (2021)
-
Femtosecond X-ray emission study of the spin cross-over dynamics in haem proteins
Nature Communications (2020)
-
Multidimensional Vibrational Coherence Spectroscopy
Topics in Current Chemistry (2018)