Abstract
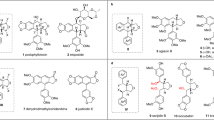
Crude oil currently provides much of the world's energy, but it is also the source of many feedstock chemicals. Methodology for the conversion of biomass into useful chemicals has often focused on either complete deoxygenation or the production of high-volume platform chemicals. Here, we describe the chemoselective partial reduction of silyl-protected C6O6-derived polyols to produce a diverse set of oxygen-functionalized chiral synthons. The combination of B(C6F5)3 and a tertiary silane efficiently generates a reactive equivalent of an electrophilic silylium ion (R3Si+) and a hydride (H−) reducing agent. The mechanism of oxygen loss does not involve a dehydrative elimination and thus avoids ablation of stereochemistry. Neighbouring group participation and the formation of cyclic intermediates is key to achieving selectivity in these reactions and, where both primary and secondary C–O bonds are present, the mechanism allows further control. The method provides—in one or two synthetic steps—highly improved syntheses of many C6On synthons as well as several previously undescribed products.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
11 August 2015
In the original graphical abstract for this Article, an in-house error meant that an incorrect intermediate was shown; this has now been corrected in the online versions.
20 August 2015
Nature Chemistry 7, 576–581 (2015); published online 23 June 2015; corrected after print 11 August 2015. In the original graphical abstract for this Article, an in-house error meant that an incorrect intermediate was shown; this has now been corrected in the online versions, and should have appearedas shown below.
References
Corma, A., Iborra, S. & Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 107, 2411–2502 (2007).
Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 41, 1538–1558 (2012).
Luterbacher, J. S., Alonso, D. M. & Dumesic, J. A. Targeted chemical upgrading of lignocellulosic biomass to platform molecules. Green Chem. 16, 4816–4838 (2014).
Tuck, C. O., Pérez, E., Horváth, I. T., Sheldon, R. A. & Poliakoff, M. Valorization of biomass: deriving more value from waste. Science 337, 695–699 (2012).
Ruppert, A. M., Weinberg, K. & Palkovits, R. Hydrogenolysis goes bio: from carbohydrates and sugar alcohols to platform chemicals. Angew. Chem. Int. Ed. 51, 2564–2601 (2012).
De Souza, R. O. M. A., Miranda, L. S. M. & Luque, R. Bio(chemo)technological strategies for biomass conversion into bioethanol and key carboxylic acids. Green Chem. 16, 2386–2405 (2014).
van Putten, R. J. et al. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 113, 1499–1597 (2013).
Mascal, M. & Nikitin, E. B. Direct, high-yield conversion of cellulose into biofuel. Angew. Chem. Int. Ed. 47, 7924–7926 (2008).
Bond, J. Q. et al. Production of renewable jet fuel range alkanes and commodity chemicals from integrated catalytic processing of biomass. Energ. Environ. Sci. 7, 1500–1523 (2014).
Bozell, J. J. & Petersen, G. R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy's ‘Top 10’ revisited. Green Chem. 12, 539–554 (2010).
Hollingsworth, R. I. & Wang, G. Toward a carbohydrate-based chemistry: progress in the development of general-purpose chiral synthons from carbohydrates. Chem. Rev. 100, 4267–4282 (2000).
Schlaf, M. Selective deoxygenation of sugar polyols to alpha,omega-diols and other oxygen content reduced materials—a new challenge to homogeneous ionic hydrogenation and hydrogenolysis catalysis. Dalton Trans. 4645–4653 (2006).
Robles, O. & Romo, D. Chemo- and site-selective derivatizations of natural products enabling biological studies. Nat. Prod. Rep. 31, 318–334 (2014).
Mahatthananchai, J., Dumas, A. M. & Bode, J. W. Catalytic selective synthesis. Angew. Chem. Int. Ed. 51, 10954–10990 (2012).
Han, S. & Miller, S. J. Asymmetric catalysis at a distance: catalytic, site-selective phosphorylation of teicoplanin. J. Am. Chem. Soc. 135, 12414–12421 (2013).
Dechert-Schmitt, A. M. R., Schmitt, D. C. & Krische, M. J. Protecting-group-free diastereoselective C–C coupling of 1,3-glycols and allyl acetate through site-selective primary alcohol dehydrogenation. Angew. Chem. Int. Ed. 52, 3195–3198 (2013).
Gouliaras, C., Lee, D., Chan, L. N. & Taylor, M. S. Regioselective activation of glycosyl acceptors by a diarylborinic acid-derived catalyst. J. Am. Chem. Soc. 133, 13926–13929 (2011).
Kawabata, T., Muramatsu, W., Nishio, T., Shibata, T. & Schedel, H. A catalytic one-step process for the chemo- and regioselective acylation of monosaccharides. J. Am. Chem. Soc. 129, 12890–12895 (2007).
McLaughlin, M. P., Adduci, L. L., Becker, J. J. & Gagné, M. R. Iridium-catalyzed hydrosilylative reduction of glucose to hexane(s). J. Am. Chem. Soc. 135, 1225–1227 (2013).
Adduci, L. L., McLaughlin, M. P., Bender, T. A., Becker, J. J. & Gagné, M. R. Metal-free deoxygenation of carbohydrates. Angew. Chem. Int. Ed. 53, 1646–1649 (2014).
Robert, T. & Oestreich, M. SiH bond activation: bridging Lewis acid catalysis with Brookhart's iridium(III) pincer complex and B(C6F5)3 . Angew. Chem. Int. Ed. 52, 5216–5218 (2013).
Gevorgyan, V., Rubin, M., Benson, S., Liu, J-X. & Yamamoto, Y. A novel B(C6F5)3-catalyzed reduction of alcohols and cleavage of aryl and alkyl ethers with hydrosilanes. J. Org. Chem. 65, 6179–6186 (2000).
Gevorgyan, V., Rubin, M., Liu, J. X. & Yamamoto, Y. A direct reduction of aliphatic aldehyde, acyl chloride, ester, and carboxylic functions into a methyl group. J. Org. Chem. 66, 1672–1675 (2001).
Parks, D. J., Blackwell, J. M. & Piers, W. E. Studies on the mechanism of B(C6F5)3-catalyzed hydrosilation of carbonyl functions. J. Org. Chem. 65, 3090–3098 (2000).
Parks, D. J. & Piers, W. E. Tris(pentafluorophenyl)boron-catalyzed hydrosilation of aromatic aldehydes, ketones, and esters. J. Am. Chem. Soc. 118, 9440–9441 (1996).
Chandrasekhar, S., Reddy, C. R. & Babu, B. N. Rapid defunctionalization of carbonyl group to methylene with polymethylhydrosiloxane-B(C6F5)3 . J. Org. Chem. 67, 9080–9082 (2002).
Nimmagadda, R. D. & McRae, C. A novel reduction reaction for the conversion of aldehydes, ketones and primary, secondary and tertiary alcohols into their corresponding alkanes. Tetrahedron Lett. 47, 5755–5758 (2006).
Oestreich, M., Hermke, J. & Mohr, J. A unified survey of Si–H and H–H bond activation catalysed by electron-deficient boranes. Chem. Soc. Rev. 44, 2202–2220 (2015).
Houghton, A. Y., Hurmalainen, J., Mansikkamäki, A., Piers, W. E. & Tuononen, H. M. Direct observation of a borane–silane complex involved in frustrated Lewis-pair-mediated hydrosilylations. Nature Chem. 6, 983–988 (2014).
Rendler, S. & Oestreich, M. Conclusive evidence for an SN2-Si mechanism in the B(C6F5)3-catalyzed hydrosilylation of carbonyl compounds: implications for the related hydrogenation. Angew. Chem. Int. Ed. 47, 5997–6000 (2008).
Haskins, W. T., Hann, R. M. & Hudson, C. S. 2,3,4,5-Dimethylene-D-mannitol and a second dimethylene-D-mannitol. J. Am. Chem. Soc. 65, 67–70 (1943).
Zissis, E. & Richtmyer, N. K. The preparation of 1,6-didesoxy-D-altritol, 1,6-didesoxygalactitol and 1,6-didesoxy-L-mannitol. J. Am. Chem. Soc. 74, 4373–4377 (1952).
Park, C. Y., Kim, B. M. & Sharpless, K. B. Catalytic osmylation of conjugated dienes : a one-pot stereoselective synthesis of polyols. Tetrahedron Lett. 32, 1003–1006 (1991).
Higashibayashi, S., Czechtizky, W., Kobayashi, Y. & Kishi, Y. Universal NMR databases for contiguous polyols. J. Am. Chem. Soc. 125, 14379–14393 (2003).
Yang, J., White, P. S. & Brookhart, M. Scope and mechanism of the iridium-catalyzed cleavage of alkyl ethers with triethylsilane. J. Am. Chem. Soc. 130, 17509–17518 (2008).
Reed, C. A. The silylium ion problem, R3Si+. Bridging organic and inorganic chemistry. Acc. Chem. Res. 31, 325–332 (1998).
Douvris, C., Nagaraja, C. M., Chen, C. H., Foxman, B. M. & Ozerov, O. V. Hydrodefluorination and other hydrodehalogenation of aliphatic carbon–halogen bonds using silylium catalysis. J. Am. Chem. Soc. 132, 4946–4953 (2010).
Nava, M. & Reed, C. A. Triethylsilyl perfluoro-tetraphenylborate, [Et3Si+][F20BPh4−], a widely used nonexistent compound. Organometallics 30, 4798–4800 (2011).
Connelly, S. J., Kaminsky, W. & Heinekey, D. M. Structure and solution reactivity of (triethylsilylium)triethylsilane cations. Organometallics 32, 7478–7481 (2013).
Tantillo, D. J. The carbocation continuum in terpene biosynthesis—where are the secondary cations? Chem. Soc. Rev. 39, 2847–2854 (2010).
Rose, M. & Palkovits, R. Isosorbide as a renewable platform chemical for versatile applications—quo vadis? ChemSusChem 5, 167–176 (2012).
Acknowledgements
The authors acknowledge the Department of Energy (DE-FG02-05ER15630) for financial support.
Author information
Authors and Affiliations
Contributions
L.L.A., T.A.B. and M.R.G. conceived and designed the experiments. L.L.A., T.A.B. and J.A.D. performed the experiments. All co-authors participated in the process of data analysis and the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5425 kb)
Rights and permissions
About this article
Cite this article
Adduci, L., Bender, T., Dabrowski, J. et al. Chemoselective conversion of biologically sourced polyols into chiral synthons. Nature Chem 7, 576–581 (2015). https://doi.org/10.1038/nchem.2277
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2277
This article is cited by
-
Catalytic amidation of natural and synthetic polyol esters with sulfonamides
Nature Communications (2019)
-
Late-stage chemoselective functional-group manipulation of bioactive natural products with super-electrophilic silylium ions
Nature Chemistry (2018)
-
Homogeneous catalysis for the production of low-volume, high-value chemicals from biomass
Nature Reviews Chemistry (2018)
-
Borane catalysed ring opening and closing cascades of furans leading to silicon functionalized synthetic intermediates
Nature Communications (2016)
-
Erratum: Chemoselective conversion of biologically sourced polyols into chiral synthons
Nature Chemistry (2015)