Abstract
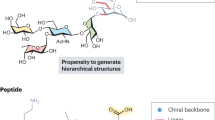
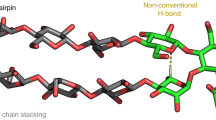
The ab initio design of synthetic molecular receptors for a specific biomolecular guest remains an elusive objective, particularly for targets such as monosaccharides, which have very close structural analogues. Here we report a powerful approach to produce receptors with very high selectivity for specific monosaccharides and, as a demonstration, we develop a foldamer that selectively encapsulates fructose. The approach uses an iterative design process that exploits the modular structure of folded synthetic oligomer sequences in conjunction with molecular modelling and structural characterization to inform subsequent refinements. Starting from a first-principles design taking size, shape and hydrogen-bonding ability into account and using the high predictability of aromatic oligoamide foldamer conformations and their propensity to crystallize, a sequence that binds to β-D-fructopyranose in organic solvents with atomic-scale complementarity was obtained in just a few iterative modifications. This scheme, which mimics the adaptable construction of biopolymers from a limited number of monomer units, provides a general protocol for the development of selective receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lokey, R. S. & Iverson, B. L. Synthetic molecules that fold into a pleated secondary structure in solution. Nature 375, 303–305 (1995).
Appella, D. H. et al. Residue-based control of helix shape in β-peptide oligomers. Nature 387, 381–384 (1997).
Nelson, J. C., Saven, J. G., Moore, J. S. & Wolynes, P. G. Solvophobically driven folding of nonbiological oligomers. Science 277, 1793–1796 (1997).
Hamuro, Y., Geib, S. J. & Hamilton, A. D. Oligoanthranilamides. Non-peptide subunits that show formation of specific secondary structure. J. Am. Chem. Soc. 118, 7529–7541 (1996).
Cuccia, L. A., Lehn, J-M., Homo, J-C. & Schmutz, M. Encoded helical self-organization and self-assembly into helical fibers of an oligoheterocyclic pyridine–pyridazine molecular strand. Angew. Chem. Int. Ed. 39, 233–237 (2000).
Berl, V., Huc, I., Khoury, R., Krische, M. & Lehn, J-M. Interconversion of single and double helices formed from synthetic molecular strands. Nature 407, 720–723 (2000).
Zhu, J. A. et al. New class of folding oligomers: crescent oligoamides. J. Am. Chem. Soc. 122, 4219–4220 (2000).
Guichard, G., Huc, I. Synthetic foldamers. Chem. Commun. 47, 5933–5941 (2011).
White, S., Szewczyk, J. W., Turner, J. M., Baird, E. E. & Dervan, P. B. Recognition of the four Watson–Crick base pairs in the DNA minor groove by synthetic ligands. Nature 391, 468–471 (1998).
Davis, A. P. Sticking to sugars. Nature 464, 169–170 (2010).
Kubik, S. Synthetic lectins. Angew. Chem. Int. Ed. 48, 1722–1725 (2009).
Huc, I. Aromatic oligoamide foldamers. Eur. J. Org. Chem. 1, 17–29 (2004).
Zhang, D-W., Zhao, X., Hou, J-L. & Li, Z-T. Aromatic amide foldamers: structures, properties, and functions. Chem. Rev. 112, 5271–5316 (2012).
Klein, E., Ferrand, Y., Barwell, N. P. & Davis, A. P. Solvent effects in carbohydrate binding by synthetic receptors: implications for the role of water in natural carbohydrate recognition. Angew. Chem. Int. Ed. 47, 2693–2696 (2008).
Ferrand, Y., Crump, M. P. & Davis, A. P. A synthetic lectin analog for biomimetic disaccharide recognition. Science 318, 619–622 (2007).
Ferrand, Y. et al. A synthetic lectin for O-linked β-N-acetylglucosamine. Angew. Chem. Int. Ed. 48, 1775–1779 (2009).
James, T. D., Sandanayake, K. R. A. S. & Shinkai, S. Chiral discrimination of monosaccharides using a fluorescent molecular sensor. Nature 374, 345–347 (1994).
James, T. D., Phillips, M. D. & Shinkai, S. Boronic Acids in Saccharide Recognition (RSC Publishing, 2006).
Davis, A. P. & Wareham, R. S. Carbohydrate recognition through noncovalent interactions: a challenge for biomimetic and supramolecular chemistry. Angew. Chem. Int. Ed. 38, 2978–2996 (1999).
Walker, D. B., Joshi, G. & Davis, A. P. Progress in biomimetic carbohydrate recognition. Cell. Mol. Life Sci. 66, 3177–3191 (2009).
Kobayashi, K., Asakawa, Y., Kato, Y. & Aoyama, Y. Complexation of hydrophobic sugars and nucleosides in water with tetrasulfonate derivatives of resorcinol cyclic tetramer having a polyhydroxy aromatic cavity importance of guest–host CH–π interaction. J. Am. Chem. Soc. 114, 10307–10313 (1992).
Das, G. & Hamilton, A. D. Molecular recognition of carbohydrates: strong binding of alkyl glycosides by phosphonate derivatives. J. Am. Chem. Soc. 116, 11139–11140 (1994).
Anderson, S., Neidlein, U., Gramlich, V. & Diederich, F. A new family of chiral binaphthyl-derived cyclophane receptors: complexation of pyranosides. Angew. Chem. Int. Ed. 34, 1596–1600 (1995).
Davis, A. P. & Wareham, R. S. A tricyclic polyamide receptor for carbohydrates in organic media. Angew. Chem. Int. Ed. 37, 2270–2273 (1998).
Bitta, J. & Kubik, S. Cyclic hexapeptides with free carboxylate groups as new receptors for monosaccharides. Org. Lett. 3, 2637–2640 (2001).
Mazik, M., Cavga, H. & Jones, P. G. Molecular recognition of carbohydrates with artificial receptors: mimicking the binding motifs found in the crystal structures of protein–carbohydrate complexes. J. Am. Chem. Soc. 127, 9045–9052 (2005).
Ardá, A. et al. A chiral pyrrolic tripodal receptor enantioselectively recognizes β-mannose and β-mannosides. Chem. Eur. J. 16, 414–418 (2010).
Inouye, M., Waki, M. & Abe, H. Saccharide-dependent induction of chiral helicity in achiral synthetic hydrogen-bonding oligomers. J. Am. Chem. Soc. 126, 2022–2027 (2004).
Hou, J-L. et al. Hydrogen bonded oligohydrazide foldamers and their recognition for saccharides. J. Am. Chem. Soc. 126, 12386–12394 (2004).
Pal, A., Bérubé, M. & Hall, D. G. Design, synthesis, and screening of a library of peptidyl bis-boroxoles as low molecular weight receptors for complex oligosaccharides in water: identification of a receptor for the tumour marker TF-antigen. Angew. Chem. Int. Ed. 49, 1492–1495 (2010).
Bao, C. et al. Converting sequences of aromatic amino acid monomers into functional three-dimensional structures: second-generation helical capsules. Angew. Chem. Int. Ed. 47, 4153–4156 (2008).
Hua, Y., Liu, Y., Chen, C-H. & Flood, A. H. Hydrophobic collapse of foldamer capsules drives picomolar-level chloride binding in aqueous acetonitrile solutions. J. Am. Chem. Soc. 135, 14401–14412 (2013).
Ajami, D. & Rebek, J. J. Compressed alkanes in reversible encapsulation complexes. Nature Chem. 1, 87–90 (2009).
Sawada, T., Yoshizawa, M., Sato, S. & Fujita, M. Minimal nucleotide duplex formation in water through enclathration in self-assembled hosts. Nature Chem. 1, 53–56 (2009).
Mugridge, J. S., Rudi van Eldik, A. Z., Bergman, R. G. & Raymond, K. N. Solvent and pressure effects on the motions of encapsulated guests: tuning the flexibility of a supramolecular host. J. Am Chem. Soc. 135, 4299–4306 (2013).
Ferrand, Y. et al. Long-range effects on the capture and release of a chiral guest by a helical molecular capsule. J. Am. Chem. Soc. 134, 11282–11288 (2012).
Qi, T., Deschrijver, T. & Huc, I. Large-scale and chromatography-free synthesis of an octameric quinoline-based aromatic amide helical foldamer. Nature Protoc. 8, 693–708 (2013).
Lemieux, R. U. The origin of the specificity in the recognition of oligosaccharides by proteins. Chem. Soc. Rev. 18, 347–374 (1989).
Cocinero, E. J. et al. Free fructose is conformationally locked. J. Am. Chem. Soc. 135, 2845–2852 (2013).
Pentelute, B. L. et al. X-ray structure of snow flea antifreeze protein determined by racemic crystallization of synthetic protein enantiomers. J. Am. Chem. Soc. 130, 9695–9701 (2008).
Lautrette, G. et al. Structure elucidation of host–guest complexes of tartaric and malic acid by quasi-racemic crystallography. Angew. Chem. Int. Ed. 52, 11517–11520 (2013).
Çarcabal, P. et al. Hydrogen bonding and cooperativity in isolated and hydrated sugars: mannose, galactose, glucose, and lactose. J. Am. Chem. Soc. 127, 11414–11425 (2005).
Doores, K. J. et al. A nonself sugar mimic of the HIV glycan shield shows enhanced antigenicity. Proc. Natl Acad. Sci. USA 107, 17107–17112 (2010).
Mecozzi, S. & Rebek J. Jr The 55% solution: a formula for molecular recognition in the liquid state. Chem. Eur. J. 4, 1016–1022 (1998).
Tanatani, A., Mio, M. J. & Moore, J. S. Chain length-dependent affinity of helical foldamers for a rodlike guest. J. Am. Chem. Soc. 123, 1792–1793 (2001).
Singleton, M. L. et al. Synthesis of 1,8-diazaanthracenes as building blocks for internally functionalized aromatic oligoamide foldamers. J. Org. Chem. 79, 2115–2122 (2014).
Corbett, P. T., Otto, S. & Sanders, J. K. M. Correlation between host–guest binding and host amplification in simulated dynamic combinatorial libraries. Chem. Eur. J. 10, 3139–3143 (2004).
Laskowski, R. A. SURFNET: a program for visualizing molecular surfaces, cavities, and intermolecular interactions. J. Mol. Graph. 13, 323–330 (1995).
Acknowledgements
This work was supported by the Agence Nationale de la Recherche (project no. ANR-09-BLAN-0082-01, post-doctoral fellowship to N.C.), by the Conseil Interprofessionnel du Vin de Bordeaux (predoctoral fellowship to G.L.) and by the European Research Council under the European Union's Seventh Framework Programme (grant agreement no. ERC-2012-AdG-320892, post-doctoral fellowship to G.L.). The authors thank A. Kendhale and C. Blum for preliminary investigations on the preparation of ‘H’ monomers, the IR-RMN-THC Fr3050 CNRS for high-field NMR time, J-L. Ferrer for beamtime and help during data collection on FIP-BM30A at the European Synchrotron Radiation Facility and P. Nowak (University of Gröningen) for calculations using the DCLSim software.
Author information
Authors and Affiliations
Contributions
N.C. and G.L. synthesized all new compounds. N.C., Y.F., G.L. and C.D.M. carried out solution studies. B.K. collected X-ray data and solved the crystal structures. M.L. performed modelling studies. I.H., D.D. and Y.F. designed the study. I.H., Y.F. and C.D.M. co-wrote the manuscript. All authors discussed the results and commented on the manuscript. N.C. and Y.F. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 10488 kb)
Supplementary movie
Supplementary movie (MP4 16206 kb)
Supplementary information
Crystallographic data for host-guest complex 1⊃β-7a (CIF 3372 kb)
Supplementary information
Crystallographic data for host-guest complex 1⊃α-8a (CIF 6224 kb)
Supplementary information
Crystallographic data for host-guest complex 1⊃β-9a (CIF 12211 kb)
Supplementary information
Crystallographic data for host-guest complex 1⊃α-13a (CIF 7306 kb)
Supplementary information
Crystallographic data for host-guest complex 3⊃β-7a (CIF 3543 kb)
Supplementary information
Crystallographic data for host-guest complex 5⊃β-7a (CIF 3072 kb)
Rights and permissions
About this article
Cite this article
Chandramouli, N., Ferrand, Y., Lautrette, G. et al. Iterative design of a helically folded aromatic oligoamide sequence for the selective encapsulation of fructose. Nature Chem 7, 334–341 (2015). https://doi.org/10.1038/nchem.2195
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2195
This article is cited by
-
Complexation-driven assembly of imine-linked helical receptors showing adaptive folding and temperature-dependent guest selection
Nature Communications (2024)
-
Foldamers reveal and validate therapeutic targets associated with toxic α-synuclein self-assembly
Nature Communications (2022)
-
De novo design of discrete, stable 310-helix peptide assemblies
Nature (2022)
-
Cucurbit[n]urils (n = 7, 8) can strongly bind neutral hydrophilic molecules in water
Science China Chemistry (2022)
-
A versatile living polymerization method for aromatic amides
Nature Chemistry (2021)