Abstract
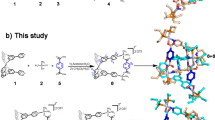
Self-assembly by means of coordinative bond formation has opened up opportunities for the high-yield synthesis of molecules with complex topologies. However, the preparation of purely covalent molecular architectures in aqueous media has remained a challenging task. Here, we present the preparation of a three-dimensional catenane through a self-assembly process that relies on the formation of dynamic hydrazone linkages in an acidic aqueous medium. The quantitative synthesis process and the mechanically interlocked structure of the resulting catenane were established by NMR spectroscopy, mass spectrometry, X-ray crystallography and HPLC studies. In addition, the labile hydrazone linkages of the individual [2]catenane components may be ‘locked’ by increasing the pH of the solution, yielding a relatively kinetically stable molecule. The present study thus details a simple approach to the creation and control of complex molecular architectures under reaction conditions that mimic biological milieux.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hudson, B. & Vinograd, J. Catenated circular DNA molecules in HeLa cell mitochondria. Nature 216, 647–652 (1967).
Clayton, D. A. & Vinograd, J. Circular dimer and catenate forms of mitochondrial DNA in human leukaemic leucocytes. Nature 216, 652–657 (1967).
Duda, R. L. Protein chainmail: catenanted protein in viral capsids. Cell 94, 55–60 (1998).
Rosengren, K. J. et al. Microcin J25 has a threaded sidechain-to-backbone ring structure and not a head-to-tail cyclized backbone. J. Am. Chem. Soc. 125, 12464–12474 (2003).
Takusagawa, F. & Kamitori, S. A real knot in protein. J. Am. Chem. Soc. 118, 8945–8946 (1996).
Mallam, A. L., Morris, E. R. & Jackson, S. E. Exploring knotting mechanisms in protein folding. Proc. Natl Acad. Sci. USA 105, 18740–18745 (2008).
Amabilino, D. B. & Stoddart, J. F. Interlocked and intertwined structures and superstructures. Chem. Rev. 95, 2725–2828 (1995).
Dietrich-Buchecker, C. O. & Sauvage, J. P. Une nouvelle famille de molecules: les metallo-catenanes. Tetrahedron Lett. 24, 5095–5098 (1983).
Lehn, J. Supramolecular chemistry. Science 260, 1762–1763 (1993).
Fyfe, M. C. T. & Stoddart, J. F. Synthetic supramolecular chemistry. Acc. Chem. Res. 30, 393–401 (1997).
Sun, Q. F. et al. Self-assembled M24L48 polyhedra and their sharp structural switch upon subtle ligand variation. Science 328, 1144–1147 (2010).
Chichak, K. S. et al. Molecular Borromean rings. Science 304, 1308–1312 (2004).
Pentecost, C. D. et al. A molecular Solomon link. Angew. Chem. Int. Ed. 46, 218–222 (2007).
Ronson, T. K. et al. Stellated polyhedral assembly of a topologically complicated Pd4L4 ‘Solomon cube’. Nature Chem. 1, 212–216 (2009).
Ayme, J.-F. et al. Lanthanide template synthesis of a molecular trefoil knot. J. Am. Chem. Soc. 136, 13142–13145 (2014).
Guo, J., Mayers, P. C., Breault, G. A. & Hunter, C. A. Synthesis of a molecular trefoil knot by folding and closing on an octahedral coordination template. Nature Chem. 2, 218–222 (2010).
Ayme, J.-F. et al. A synthetic molecular pentafoil knot. Nature Chem. 4, 15–20 (2012).
Leigh, D. A., Pritchard, R. G. & Stephens, A. J. A star of David catenane. Nature Chem. 6, 978–982 (2014).
Fujita, M., Fujita, N., Ogura, K. & Yamaguchi, K. Spontaneous assembly of ten components into two interlocked, identical coordination cages. Nature 400, 52–55 (1999).
Fukuda, M., Sekiya, R. & Kuroda, R. A quadruply stranded metallohelicate and its spontaneous dimerization into an interlocked metallohelicate. Angew. Chem. Int. Ed. 47, 706–710 (2008).
Westcott, A., Fisher, J., Harding, L. P., Rizkallah, P. & Hardie, M. J. Self-assembly of a 3-D triply interlocked chiral [2]catenane. J. Am. Chem. Soc. 130, 2950–2951 (2008).
Hasell, T. et al. Triply interlocked covalent organic cages. Nature Chem. 2, 750–755 (2010).
Zhang, G., Presly, O., White, F., Oppel, I. M. & Mastalerz, M. A shape-persistent quadruply interlocked giant cage catenane with two distinct pores in the solid state. Angew. Chem. Int. Ed. 53, 5126–5130 (2014).
Ponnuswamy, N., Cougnon, F. B. L., Clough, J. M., Pantoş, G. D. & Sanders, J. K. M. Discovery of an organic trefoil knot. Science 338, 783–785 (2012).
Wang, L., Vysotsky, M. O., Bogdan, A., Bolte, M. & Böhmer, V. Multiple catenanes derived from calix[4]arenes. Science 304, 1312–1314 (2004).
Li, Y. et al. Sulfate anion templated synthesis of a triply interlocked capsule. Chem. Commun. 7134–7136 (2009).
Orrillo, A. G., Escalante, A. M. & Furlan, R. L. E. Covalent double level dynamic combinatorial libraries: selectively addressable exchange processes. Chem. Commun. 5298–5300 (2008).
Rodriguez-Docampo, Z. & Otto, S. Orthogonal or simultaneous use of disulfide and hydrazone exchange in dynamic covalent chemistry in aqueous solution. Chem. Commun. 5301–5303 (2008).
Acknowledgements
This work was supported by the US National Science Foundation (grant no. CHE-1402004) and the Robert A. Welch Foundation (F-1018). H.L. acknowledges support from a Chinese Government Award under the ‘Outstanding Self-Financed Student Abroad and the Thousand Young Talents Plan’. X.L. acknowledges support from the National Science Foundation (CHE-1506722) and the PREM Center for Interfaces (DMR-1205670).
Author information
Authors and Affiliations
Contributions
H.L. and J.L.S. conceived the project. H.L. and J.L.S. prepared the manuscript. H.L., H.Z. and A.D.L. synthesized the molecules studied in this work. V.M.L. solved the crystal structure. H.L., M.W. and X.L. were responsible for MS studies. H.L. performed the NMR spectroscopic and HPLC investigations.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 16421 kb)
Supplementary information
Crystallographic data for compound C6+.6Cl- (CIF 2075 kb)
Rights and permissions
About this article
Cite this article
Li, H., Zhang, H., Lammer, A. et al. Quantitative self-assembly of a purely organic three-dimensional catenane in water. Nature Chem 7, 1003–1008 (2015). https://doi.org/10.1038/nchem.2392
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2392
This article is cited by
-
Dimeric and trimeric catenation of giant chiral [8 + 12] imine cubes driven by weak supramolecular interactions
Nature Chemistry (2023)
-
The sharp structural switch of covalent cages mediated by subtle variation of directing groups
Nature Communications (2023)
-
A trefoil knot self-templated through imination in water
Nature Communications (2022)
-
Self-assembled poly-catenanes from supramolecular toroidal building blocks
Nature (2020)
-
A cyclic bis[2]catenane metallacage
Nature Communications (2020)