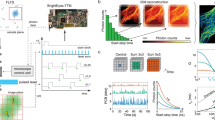
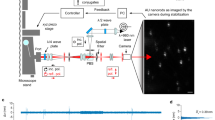
Abstract
Single-molecule localization microscopy is used to construct super-resolution images, but generally requires prior intense laser irradiation and in some cases additives, such as thiols, to induce on–off switching of fluorophores. These requirements limit the potential applications of this methodology. Here, we report a first-in-class spontaneously blinking fluorophore based on an intramolecular spirocyclization reaction. Optimization of the intramolecular nucleophile and rhodamine-based fluorophore (electrophile) provide a suitable lifetime for the fluorescent open form, and equilibrium between the open form and the non-fluorescent closed form. We show that this spontaneously blinking fluorophore is suitable for single-molecule localization microscopy imaging deep inside cells and for tracking the motion of structures in living cells. We further demonstrate the advantages of this fluorophore over existing methodologies by applying it to nuclear pore structures located far above the coverslip with a spinning-disk confocal microscope and for repetitive time-lapse super-resolution imaging of microtubules in live cells for up to 1 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hell, S. W. Far-field optical nanoscopy. Science 316, 1153–1158 (2007).
Huang, B., Babcock, H. & Zhuang, X. Breaking the diffraction barrier: super-resolution imaging of cells. Cell 143, 1047–1058 (2010).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Hess, S. T., Girirajan, T. P. K. & Mason, M. D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 91, 4258–4272 (2006).
Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nature Methods 3, 793–796 (2006).
Folling, J. et al. Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nature Methods 5, 943–945 (2008).
Heilemann, M. et al. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Ed. 47, 6172–6176 (2008).
Vogelsang, J. et al. Make them blink: probes for super-resolution microscopy. ChemPhysChem 11, 2475–2490 (2010).
van de Linde, S., Heilemann, M. & Sauer, M. Live-cell super-resolution imaging with synthetic fluorophores. Annu. Rev. Phys. Chem. 63, 519–540 (2012).
Steinhauer, C., Forthmann, C., Vogelsang, J. & Tinnefeld, P. Superresolution microscopy on the basis of engineered dark states. J. Am. Chem. Soc. 130, 16840–16841 (2008).
Schwering, M. et al. Far-field nanoscopy with reversible chemical reactions. Angew. Chem. Int. Ed. 50, 2940–2945 (2011).
van de Linde, S. et al. Direct stochastic optical reconstruction microscopy with standard fluorescent probes. Nature Protoc. 6, 991–1009 (2011).
Dempsey, G. T., Vaughan, J. C., Chen, K. H., Bates, M. & Zhuang, X. Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nature Methods 8, 1027–1036 (2011).
Wombacher, R. et al. Live-cell super-resolution imaging with trimethoprim conjugates. Nature Methods 7, 717–719 (2010).
Klein, T. et al. Live-cell dSTROM with SNAP-tag fusion proteins. Nature Methods 8, 7–9 (2011).
Lukinavičius, G. et al. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nature Chem. 5, 132–139 (2013).
Kim, H. N., Lee, M. H., Kim, H. J., Kim, J. S. & Yoon, J. A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 37, 1465–1472 (2008).
Kenmoku, S., Urano, Y., Kojima, H. & Nagano, T. Development of a highly specific rhodamine-based fluorescence probe for hypochlorous acid and its application to real-time imaging of phagocytosis. J. Am. Chem. Soc. 129, 7313–7318 (2007).
Koide, Y., Urano, Y., Hanaoka, K., Terai, T. & Nagano, T. Development of an Si-rhodamine-based far-red to near-infrared fluorescence probe selective for hypochlorous acid and its applications for biological imaging. J. Am. Chem. Soc. 133, 5680–5682 (2011).
Kamiya, M. et al. β-Galactosidase fluorescence probe with improved cellular accumulation based on a spirocyclized rhodol scaffold. J. Am. Chem. Soc. 133, 12960–12963 (2011).
Sakabe, M. et al. Rational design of highly sensitive fluorescence probes for protease and glycosidase based on precisely controlled spirocyclization. J. Am. Chem. Soc. 135, 409–414 (2013).
Fölling, J. et al. Photochromic rhodamines provide nanoscopy with optical sectioning. Angew. Chem.Int. Ed. 46, 6266–6270 (2007).
Belov, V. N., Bossi, M. L., Fölling, J., Boyarskiy, V. P. & Hell, S. W. Rhodamine spiroamides for multicolor single-molecule switching fluorescent nanoscopy. Chem. Eur. J. 15, 10762–10776 (2009).
Fu, M., Xiao, Y., Qian, X., Zhao, D. & Xu, Y. A design concept of long-wavelength fluorescent analogs of rhodamine dyes: replacement of oxygen with silicon atom. Chem. Commun. 1780–1782 (2008).
Koide, Y., Urano, Y., Hanaoka, K., Terai, T. & Nagano, T. Evolution of group 14 rhodamines as platforms for near-infrared fluorescence probes utilizing photoinduced electron transfer. ACS Chem. Biol. 6, 600–608 (2011).
Corrie, J. E. T. et al. Ring-chain interconversion of sulforhodamine-amine conjugates involves an unusually labile C–N bond and allows measurement of sulfonamide ionization kinetics. J. Phys. Org. Chem. 21, 286–298 (2008).
Katayama, H., Yamamoto, A., Mizushima, N., Yoshimori, T. & Miyawaki, A. GFP-like proteins stably accumulate in lysosomes. Cell. Struct. Funct. 33, 1–12 (2008).
Jones, S. A., Shim, S.-H., He, J. & Zhuang, X. Fast, three-dimensional super-resolution imaging of live cells. Nature Methods 8, 499–505 (2011).
Quan, T., Zeng, S. & Huang, Z-L. Localization capability and limitation of electron-multiplying charge-coupled, scientific complementary metal–oxide semiconductor, and charge-coupled devices for superresolution imaging. J. Biomed. Opt. 15, 066005 (2010).
Tokunaga, M., Imamoto, N. & Sakata-Sogawa, K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nature Methods 5, 159–161 (2008).
Löschberger, A. et al. Super-resolution imaging visualizes the eightfold symmetry of gp210 proteins around the nuclear pore complex and resolves the central channel with nanometer resolution. J. Cell. Sci. 125, 570–575 (2012).
Szymborska, A. et al. Nuclear pore scaffold structure analyzed by super-resolution microscopy and particle averaging. Science 341, 655–658 (2013).
Funakoshi, T. et al. Two distinct human POM121 genes: requirement for the formation of nuclear pore complexes. FEBS Lett. 581, 4910–4916 (2007).
Maeshima, K. et al. Nuclear pore formation but not nuclear growth is governed by cyclin-dependent kinases (Cdks) during interphase. Nature Struct. Mol. Biol. 17, 1065–1071 (2010).
Hoelz, A., Debler, E. W. & Blobel, G. The structure of the nuclear pore complex. Annu. Rev. Biochem. 80, 613–643 (2011).
Hinner, M. J. & Johnsson, K. How to obtain labeled proteins and what to do with them. Curr. Opin. Biotechnol. 21, 766–776 (2010).
Los, G. V. et al. Halotag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 3, 373–382 (2008).
Acknowledgements
This research was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid for Scientific Research (KAKENHI): 20117003 and 23249004 to Y.U., 23113504 and 25113707 to M.K., 23710108 to M.C.T., 24659092, 25113723 and 25293046 to Y.O. and 21121004 to T. F.), by The Daiichi-Sankyo Foundation of Life Science (to Y.U.), by the Uehara memorial Foundation (to Y.O.), by The Mochida Memorial Foundation for Medical and Pharmaceutical Research (to M.K.) and by a JSPS stipend (to S.U.). The authors thank D. Toomre for many useful discussions, K. Johnsson and G. Lukinavičius for β-tubulin–SNAP plasmid, N. Imamoto for HeLa cell lines expressing nuclear pore proteins, R. Wong for POM121 plasmid, J. Asada and S. Dan Xu for the construction of plasmids and experimental assistance, and Y. Arai for the ImageJ script for analysing projection profiles of the localization of super-resolution images. The African green monkey kidney normal cell line Vero (JCRB0111) was obtained from the Japanese Collection of Research Bioresources (JCRB) cell bank.
Author information
Authors and Affiliations
Contributions
S.U., M.K., T.Y., Y.O., S.T. and Y.U. conducted experiments and performed analyses. S.U., M.K., Y.O. and Y.U. co-wrote the manuscript. K.S., K.O., M.C.T., T.F. and H.F. contributed materials/analysis tools. M.K. and Y.U. planned and initiated the project, designed experiments, and supervised the entire project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 3781 kb)
Supplementary information
Supplementary Movie 1 (AVI 9195 kb)
Supplementary information
Supplementary Movie 2 (AVI 2654 kb)
Supplementary information
Supplementary Movie 3 (AVI 476 kb)
Supplementary information
Supplementary Movie 4 (AVI 1657 kb)
Supplementary information
Supplementary Movie 5 (AVI 786 kb)
Rights and permissions
About this article
Cite this article
Uno, Sn., Kamiya, M., Yoshihara, T. et al. A spontaneously blinking fluorophore based on intramolecular spirocyclization for live-cell super-resolution imaging. Nature Chem 6, 681–689 (2014). https://doi.org/10.1038/nchem.2002
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2002
This article is cited by
-
A general strategy to develop fluorogenic polymethine dyes for bioimaging
Nature Chemistry (2024)
-
Bioorthogonal Chemistry in Cellular Organelles
Topics in Current Chemistry (2024)
-
Fast-exchanging spirocyclic rhodamine probes for aptamer-based super-resolution RNA imaging
Nature Communications (2023)
-
Organic fluorescent probes for live-cell super-resolution imaging
Frontiers of Optoelectronics (2023)
-
Photoswitching fingerprint analysis bypasses the 10-nm resolution barrier
Nature Methods (2022)