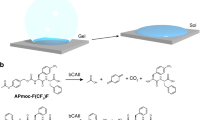
Abstract
Soft materials that exhibit stimuli-responsive behaviour under aqueous conditions (such as supramolecular hydrogels composed of self-assembled nanofibres) have many potential biological applications. However, designing a macroscopic response to structurally complex biochemical stimuli in these materials still remains a challenge. Here we show that redox-responsive peptide-based hydrogels have the ability to encapsulate enzymes and still retain their activities. Moreover, cooperative coupling of enzymatic reactions with the gel response enables us to construct unique stimuli-responsive soft materials capable of sensing a variety of disease-related biomarkers. The programmable gel–sol response (even to biological samples) is visible to the naked eye. Furthermore, we built Boolean logic gates (OR and AND) into the hydrogel–enzyme hybrid materials, which were able to sense simultaneously plural specific biochemicals and execute a controlled drug release in accordance with the logic operation. The intelligent soft materials that we have developed may prove valuable in future medical diagnostics or treatments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
de Gennes, P-G. Soft matter (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 31, 842–845 (1992).
Holtz, J. H. & Asher, S. A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 389, 829–832 (1997).
Qiu, Y. & Park, K. Environment-sensitive hydrogel for drug delivery. Adv. Drug Deliv. Rev. 53, 321–339 (2001).
Miyata, T., Uragami, T. & Nakamae, K. Biomolecule-sensitive hydrogel. Adv. Drug Deliv. Rev. 54, 79–98 (2002).
Lutolf, M. P. & Hubbell, J. A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnol. 23, 47–55 (2005).
Burdick, J. A. & Murphy, W. L. Moving from static to dynamic complexity in hydrogel design. Nature Commun. 3, 1269 (2012).
Alberts, B. et al. Molecular Biology of the Cell 5th edn (Garland Science, 2008).
Lehninger, A., Nelson, D. L. & Cox, M. M. Lehninger Principles of Biochemistry 5th edn (W. H. Freeman, 2008).
Alberti, K. G. M. M. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Med. 15, 539–553 (1998).
Wallace, K. L., Riedel, A. A., Joseph-Ridge, N. & Wortmann, R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J. Rheumatol. 31, 1582–1587 (2004).
Hershfielda, M. S. et al. Treating gout with pegloticase, a PEGylated urate oxidase, provides insight into the importance of uric acid as an antioxidant in vivo. Proc. Natl Acad. Sci. USA 107, 14351–14356 (2010).
Sreekumar, A. et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 457, 910–914 (2009).
Estroff, L. A. & Hamilton, A. D. Water gelation by small organic molecules. Chem. Rev. 104, 1201–1217 (2004).
de Loos, M., Feringa, B. L. & van Esch, J. H. Design and application of self-assembled low molecular weight hydrogels. Eur. J. Org. Chem. 2005, 3615–3631 (2005).
Weiss, R. G. & Terech, P. Molecular Gels: Materials with Self-Assembled Fibrillar Networks Ch. 17, Ch. 18 (Springer: 2006).
Yang, Z. & Xu, B. Supramolecular hydrogels based on biofunctional nanofibers of self-assembled small molecules. J. Mater. Chem. 17, 2385–2393 (2007).
Hirst, A. R., Escuder, B., Miravet, J. F. & Smith, D. K. High-tech applications of self-assembling supramolecular nanostructured gel-phase materials: from regenerative medicine to electronic devices. Angew. Chem. Int. Ed. 47, 8002–8018 (2008).
Kiyonaka, S. et al. Semi-wet peptide/protein array using supramolecular hydrogel. Nature Mater. 3, 58–64 (2004).
Yang, Z. & Xu, B. A simple visual assay based on small molecule hydrogels for detecting inhibitors of enzymes. Chem. Commun. 2424–2425 (2004).
Ikeda, M. et al. Montmorillonite-supramolecular hydrogel hybrid for fluorocolorimetric sensing of polyamines. J. Am. Chem. Soc. 133, 1670–1673 (2011).
Ikeda, M., Ochi, R. & Hamachi, I. Supramolecular hydrogel-based protein and chemosensor array. Lab Chip 10, 3325–3334 (2010).
Holmes, T. C. et al. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc. Natl Acad. Sci. USA 97, 6728–6733 (2000).
Silva, G. A. et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303, 1352–1355 (2004).
Haines-Butterick, L. et al. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc. Natl Acad. Sci. USA 104, 7791–7796 (2007).
Aggeli, A. et al. Responsive gels formed by the spontaneous self-assembly of peptides into polymeric β-sheet tapes. Nature 386, 259–262 (1997).
Xu, X-D. et al. Biological glucose metabolism regulated peptide self-assembly as a simple visual biosensor for glucose detection. Macromol. Rapid Commun. 33, 426–431 (2012).
Piepenbrock, M-O. M., Lloyd, G. O., Clarke, N. & Steed, J. W. Metal- and anion-binding supramolecular gels. Chem. Rev. 110, 1960–2004 (2010).
Komatsu, H. et al. Supramolecular hydrogel exhibiting four basic logic gate functions to fine-tune substance release. J. Am. Chem. Soc. 131, 5580–5585 (2009).
Kim, H-J., Lee, J-H. & Lee, M. Stimuli-responsive gels from reversible coordination polymers. Angew. Chem. Int. Ed. 44, 5810–5814 (2005).
Zhang, Y., Gu, H., Yang, Z. & Xu, B. Supramolecular hydrogels respond to ligand−receptor interaction. J. Am. Chem. Soc. 125, 13680–13681 (2003).
Zhang, X. et al. Rational design of a tetrameric protein to enhance interactions between self-assembled fibers gives molecular hydrogels. Angew. Chem. Int. Ed. 51, 4388–4392 (2012).
Yang, Z., Liang, G. & Xu, B. Enzymatic control of the self-assembly of small molecules: a new way to generate supramolecular hydrogels. Soft Matter 3, 515–520 (2007).
Ulijn, R. V. Enzyme-responsive materials: a new class of smart biomaterials. J. Mater. Chem. 16, 2217–2225 (2006).
Hirst, A. R. et al. Biocatalytic induction of supramolecular order. Nature Chem. 2, 1089–1094 (2010).
Ikeda, M., Tanida, T., Yoshii, T. & Hamachi, I. Rational molecular design of stimulus-responsive supramolecular hydrogels based on dipeptides. Adv. Mater. 23, 2819–2822 (2011).
Bowerman, C. J. & Nilsson, B. L. A reductive trigger for peptide self-assembly and hydrogelation. J. Am. Chem. Soc. 132, 9526–9527 (2010).
Miao, X. et al. Switchable catalytic activity: selenium-containing peptides with redox-controllable self-assembly properties. Angew. Chem. Int. Ed. 52, 7781–7785 (2013).
Sun, Z. et al. Ferrocenoyl phenylalanine: a new strategy toward supramolecular hydrogels with multistimuli responsive properties. J. Am. Chem. Soc. 135, 13379–13386 (2013).
Chen, L., Revel, S., Morris, K., Serpell, L. C. & Adams, D. J. Effect of molecular structure on the properties of naphthalene–dipeptide hydrogelators. Langmuir 26, 13466–13471 (2010).
Lippert, A-R., Van de Bittner, G. C. & Chang, C. J. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc. Chem. Res. 44, 793–804 (2011).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Gatenby, R. A. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nature Rev. Cancer 4, 891–899 (2004).
Morris, K. L. et al. Chemically programmed self-sorting of gelator networks. Nature Commun. 4, 1480 (2013).
Swanekamp, R. J., DiMaio, J. T. M., Bowerman, C. J. & Nilsson, B. L. Coassembly of enantiomeric amphipathic peptides into amyloid-inspired rippled β-sheet fibrils. J. Am. Chem. Soc. 134, 5556–5559 (2012).
Buerkle, L. E., Li, Z., Jamieson, A. M. & Rowan, S. J. Tailoring the properties of guanosine-based supramolecular hydrogels. Langmuir 25, 8833–8840 (2009).
Schneider, H-J., Tianjun, L., Lomadze, N. & Palm, B. Cooperativity in a chemomechanical polymer: a chemically induced macroscopic logic gate. Adv. Mater. 16, 613–615 (2004).
De Silva, A. P. & Uchiyama, S. Molecular logic and computing. Nature Nanotech. 2, 399–410 (2007).
Katz, E. & Privman, V. Enzyme-based logic systems for information processing. Chem. Soc. Rev. 39, 1835–1857 (2010).
Win, M. N. & Smolke, C. D. Higher-order cellular information processing with synthetic RNA devices. Science 322, 456–460 (2008).
Douglas, S. M., Bachelet, I. & Church, G. M. A logic-gated nanorobot for targeted transport of molecular payloads. Science 335, 831–834 (2012).
Acknowledgements
This work was supported in part by the JST (Japan Science and Technology Agency), the CREST (Core Research for Evolutionary Science and Technology) program, a Grant-in-Aid for Young Scientists (A) (No. 23681022), the Scientific Research on the Innovative Areas ‘Molecular Robotics’ (No. 25104512), the global Centre of Excellence program, ‘Integrated Materials Science’ of the Ministry of Education, Culture, Science, Sports and Technology (Japan). M.I. thanks the Tokuyama Science Foundation for financial support. We acknowledge Y. Chujo and N. Kitamura (Kyoto University) for allowing us to use the transmission electron microscope and for their support, K. Kuwata (Kyoto University) for high-resolution mass spectroscopy measurements, E. Kusaka (Kyoto University) for NMR measurements and M. Ichihashi and M. Wagatsuma (Initium, ULVAC) for quartz-crystal microbalance measurements. We thank E. Ashihara (Kyoto Pharmaceutical University) and Y. Takaoka (Kyoto University) for their help in taking blood samples. T.Y. acknowledges the JSPS (Japan Society for the Promotion of Science) Research Fellowship for Young Scientists.
Author information
Authors and Affiliations
Contributions
M.I., T.T., T.Y., K.K., S.O. and K.U. performed the experiments and M.I. and I.H. conceived the project. The paper was written by M.I. and I.H. and edited by all the co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 6044 kb)
Rights and permissions
About this article
Cite this article
Ikeda, M., Tanida, T., Yoshii, T. et al. Installing logic-gate responses to a variety of biological substances in supramolecular hydrogel–enzyme hybrids. Nature Chem 6, 511–518 (2014). https://doi.org/10.1038/nchem.1937
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1937
This article is cited by
-
Dipeptide coacervates as artificial membraneless organelles for bioorthogonal catalysis
Nature Communications (2024)
-
Synaptic transistor with multiple biological functions based on metal-organic frameworks combined with the LIF model of a spiking neural network to recognize temporal information
Microsystems & Nanoengineering (2023)
-
Emulsion-oriented assembly for Janus double-spherical mesoporous nanoparticles as biological logic gates
Nature Chemistry (2023)
-
Four distinct network patterns of supramolecular/polymer composite hydrogels controlled by formation kinetics and interfiber interactions
Nature Communications (2023)
-
Skin-Inspired Ultra-Tough Supramolecular Multifunctional Hydrogel Electronic Skin for Human–Machine Interaction
Nano-Micro Letters (2023)