Abstract
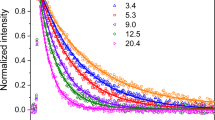
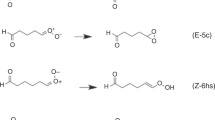
Criegee intermediates, which are carbonyl oxides produced when ozone reacts with unsaturated hydrocarbons, play an important role in the formation of OH and organic acids in the atmosphere, but they have eluded direct detection until recently. Reactions that involve Criegee intermediates are not understood fully because data based on their direct observation are limited. We used transient infrared absorption spectroscopy to probe directly the decay kinetics of formaldehyde oxide (CH2OO) and found that it reacts with itself extremely rapidly. This fast self-reaction is a result of its zwitterionic character. According to our quantum-chemical calculations, a cyclic dimeric intermediate that has the terminal O atom of one CH2OO bonded to the C atom of the other CH2OO is formed with large exothermicity before further decomposition to 2H2CO + O2(1Δg). We suggest that the inclusion of this previously overlooked rapid reaction in models may affect the interpretation of previous laboratory experiments that involve Criegee intermediates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Johnson, D. & Marston, G. The gas-phase ozonolysis of unsaturated volatile organic compounds in the troposphere. Chem. Soc. Rev. 37, 699–716 (2008).
Calvert, J. G. et al. The Mechanisms of Atmospheric Oxidation of the Alkenes 172–335 (Oxford Univ. Press, 2000).
Horie, O. & Moortgat, G. K. Gas-phase ozonolysis of alkenes. Recent advances in mechanistic investigations. Acc. Chem. Res. 31, 387–396 (1998).
Sander, W. Carbonyl oxides: zwitterions or diradicals? Angew. Chem. Int. Ed. Engl. 29, 344–354 (1990).
Bunnelle, W. H. Preparation, properties, and reactions of carbonyl oxides. Chem. Rev. 91, 335–362 (1991).
Hatakeyama, S. & Akimoto, H. Reactions of Criegee intermediates in the gas phase. Res. Chem. Intermed. 20, 503–524 (1994).
Criegee, R. & Wenner, G. Die ozonisierung des 9,10-oktalins. Liebigs Ann. Chem. 564, 9–15 (1949).
Taatjes, C. A. et al. Direct observation of the gas-phase Criegee intermediate (CH2OO). J. Am. Chem. Soc. 130, 11883–11885 (2008).
Welz, O. et al. Direct kinetic measurements of Criegee intermediate (CH2OO) formed by reaction of CH2I with O2 . Science 335, 204–207 (2012).
Beames, J. M., Liu, F., Lu, L. & Lester, M. I. Ultraviolet spectrum and photochemistry of the simplest Criegee intermediate CH2OO. J. Am. Chem. Soc. 134, 20045–20048 (2012).
Su, Y-T., Huang, Y-H., Witek, H. A. & Lee, Y-P. Infrared absorption spectrum of the simplest Criegee intermediate CH2OO. Science 340, 174–176 (2013).
Nakajima, M. & Endo, Y. Determination of the molecular structure of the simplest Criegee intermediate CH2OO. J. Chem. Phys. 139, 101103 (2013).
Nguyen, M. T., Ngyuen, T. L., Ngan, V. T. & Ngyuen, H. M. T. Heats of formation of the Criegee formaldehyde oxide and dioxirane. Chem. Phys. Lett. 448, 183–188 (2007).
Anglada, J. M., Gonzalez, J. & Torrent-Sucarrat, M. Effects of the substituents on the reactivity of carbonyl oxides. A theoretical study on the reaction of substituted carbonyl oxides with water. Phys. Chem. Chem. Phys. 13, 13034–13045 (2011).
Vereecken, L. & Francisco, J. S. Theoretical studies of atmospheric reaction mechanisms in the troposphere. Chem. Soc. Rev. 41, 6259–6293 (2012).
Cremer, D., Gauss, J., Kraka, E., Stanton, J. F. & Bartlett, R. J. A CCSD (T) investigation of carbonyl oxide and dioxirane. Equilibrium geometries, dipole moments, infrared spectra, heats of formation and isomerization energies. Chem. Phys. Lett. 209, 547–556 (1993).
Fang, D-C. & Fu, X-Y. CASSCF and CAS+1+2 studies on the potential energy surface and the rate constants for the reactions between CH2 and O2 . J. Phys. Chem. A 106, 2988–2993 (2002).
Cool, T. A., Wang, J., Nakajima, K., Taatjes, C. A. & McIlroy, A. Photoionization cross sections for reaction intermediates in hydrocarbon combustion. Int. J. Mass Spectrom. 247, 18–27 (2005).
Sander, S. P. et al. Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies (Evaluation Number 14, JPL Publication 02-25, 2003).
Mössinger, J. C., Shallcross, D. E. & Cox, R. A. UV-vis absorption cross-sections and atmospheric lifetimes of CH2Br2, CH2I2 and CH2BrI. J. Chem. Soc. Faraday Trans. 94, 1391–1396 (1998).
Vogt, R., Sander, R., Glasow, R. V. & Crutzen, P. J. Iodine chemistry and its role in halogen activation and ozone loss in the marine boundary layer: a model study. J. Atmos. Chem. 32, 375–395 (1999).
Saiz-Lopez, A. et al. Atmospheric chemistry of iodine. Chem. Rev. 112, 1773–1804 (2012).
Rienstra-Kiracofe, J. C., Allen, W. D. & Schaefer, H. F. III . The C2H5 + O2 reaction mechanism: high-level ab initio characterizations. J. Phys. Chem. A 104, 9823–9840 (2000).
Liang, Y-N., Li, J., Wang, Q-D., Wang, F. & Li, X-Y. Computational study of the reaction mechanism of the methylperoxy self-reaction. J. Phys. Chem. A 115, 13534–13541 (2011).
Vereecken, L., Harder, H. & Novelli, A. The reaction of Criegee intermediates with NO, RO2, and SO2, and their fate in the atmosphere. Phys. Chem. Chem. Phys. 14, 14682–14695 (2012).
Kee, R. J., Rupley, F. M. & Miller, J. A. Chemkin-II: A Fortran Chemical Kinetics Package for the Analysis of Gas-Phase Chemical Kinetics Sandia Report SAND89-8009B (Sandia National Laboratories, 1995).
Masaki, A., Tsunashima, S. & Washida, N. Rate constants for reactions of substituted methyl radicals (CH2OCH3, CH2NH2, CH2I, and CH2CN) with O2 . J. Phys. Chem. 99, 13126–13131 (1995).
Eskola, A. J., Wojcik-Pastuszka, D., Ratajczak E. & Timonen, R. S. Kinetics of the reactions of CH2Br and CH2I radicals with molecular oxygen at atmospheric temperatures. Phys. Chem. Chem. Phys. 8, 1416–1424 (2006).
Atkinson, R. et al. Summary of Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry, IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry (2006). http://rpw.chem.ox.ac.uk/IUPACsumm_web_latest.pdf.
Taatjes, C. A. et al. Direct measurement of Criegee intermediate (CH2OO) reactions with acetone, acetaldehyde, and hexafluoroacetone. Phys. Chem. Chem. Phys. 14, 10391–10400 (2012).
Neeb, P., Horie, O. & Moortgat, G. K. The ethane-ozone reaction in the gas phase. J. Phys. Chem. A 102, 6778–6785 (1998).
McFiggans, G. et al. Direct evidence for coastal iodine particles from Laminaria marcroalgae – linkage to emissions of molecular iodine. Atmos. Chem. Phys. 4, 701–713 (2004).
Gravestock, T. J., Blitz, M. A., Bloss, W. J. & Heard D. E. A multidimensional study of the reaction CH2I + O2: products and atmospheric implications. ChemPhysChem 11, 3928–3941 (2010).
Stone, D., Blitz, M., Daubney, L., Ingham, T. & Seakins, P. CH2OO Criegee biradical yields following photolysis of CH2I2 in O2 . Phys. Chem. Chem. Phys. 15, 19119–19124 (2013).
Cotter, E. S. N., Booth, N. J., Canosa-Mas, C. E. & Wayne, R. P. Release of iodine in the atmospheric oxidation of alkyl iodides and the fates of iodinated alkoxy radicals. Atmos. Environ. 35, 2169–2178 (2001).
Sehested, J., Ellermann, T. & Nielsen, O. J. A spectrokinetic study of CH2I and CH2IO2 radicals. Int. J. Chem. Kinet. 26, 259–272 (1994).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Dunning, T. H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90, 1007–1023 (1989).
Peterson, K. A., Shepler, B. C., Figgen, D. & Stoll, H. On the spectroscopic and thermochemical properties of ClO, BrO, IO, and their anions. J. Phys. Chem. A 110, 13877–13883 (2006).
Pople, J. A., Head-Gordon, M. & Raghavachari, K. Quadratic configuration interaction. A general technique for determining electron correlation energies. J. Chem. Phys. 87, 5968–5975 (1987).
Scuseria, G. E. & Schaefer III, H. F. Is coupled cluster singles and doubles (CCSD) more computationally intensive than quadratic configuration interaction (QCISD)? J. Chem. Phys. 90, 3700–3703 (1989).
Gonzalez, C. & Schlegel, H. B. An improved algorithm for reaction path following. J. Chem. Phys. 90, 2154–2161 (1989).
Frisch, M. J. et al. GAUSSIAN 09, Revision A02 (Gaussian, Inc., Wallingford Connecticut, 2009).
Werner, H-J. et al. MOLPRO, version 2009.1. A package of ab initio programs, http://www.molpro.net (University College Cardiff Consultants, Cardiff, UK).
Wardlaw, D. M. & Marcus, R. A. RRKM reaction rate theory for transition states of any looseness. Chem. Phys. Lett. 110, 230–234 (1984).
Wardlaw, D. M. & Marcus, R. A. Unimolecular reaction rate theory for transition states of partial looseness. II. Implementation and analysis with applications to NO2 and C2H6 dissociations. J. Chem. Phys. 83, 3462–3480 (1985).
Klippenstein, S. J. Variational optimizations in the Rice–Ramsperger–Kassel–Marcus theory calculations for unimolecular dissociations with no reverse barrier. J. Chem. Phys. 96, 367–371 (1992).
Klippenstein, S. J. & Marcus, R. A. High pressure rate constants for unimolecular dissociation/free radical recombination: determination of the quantum correction via quantum Monte Carlo path integration. J. Chem. Phys. 87, 3410–3417 (1987).
Klippenstein, S. J., Wagner, A. F., Dunbar, R. C., Wardlaw, D. M. & Robertson, S. H. VARIFLEX Version 1.00 (Argonne National Laboratory, Argonne, Illinois, 1999).
Acknowledgements
The National Science Council of Taiwan (grants NSC102-2745-M-009-001-ASP and NSC101-2113-M-009-002) and the Ministry of Education, Taiwan (‘ATU Plan’ of the National Chiao Tung University) supported this work. The National Center for High-Performance Computing provided computer time.
Author information
Authors and Affiliations
Contributions
Y-T.S. performed the experiments and analysed the data, H-Y.L. performed the kinetic simulations and R.P. performed the calculations. H.M. conceived and designed the kinetic analysis. M.C.L. conceived and designed the calculations. Y-P.L. conceived and designed the experiments and wrote a major part of the paper. H.M. and M.C.L. contributed to writing sections of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1818 kb)
Rights and permissions
About this article
Cite this article
Su, YT., Lin, HY., Putikam, R. et al. Extremely rapid self-reaction of the simplest Criegee intermediate CH2OO and its implications in atmospheric chemistry. Nature Chem 6, 477–483 (2014). https://doi.org/10.1038/nchem.1890
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1890
This article is cited by
-
Capturing primary ozonides for a syn-dihydroxylation of olefins
Nature Chemistry (2023)
-
Photodissociation pathways in the simplest Criegee intermediate: a semi-classical investigation
Journal of Chemical Sciences (2023)
-
Formation reaction mechanism and infrared spectra of anti-trans-methacrolein oxide and its associated precursor and adduct radicals
Communications Chemistry (2022)
-
Theoretical kinetics studies on the temperature and pressure dependence of the reaction of ammonia with the Criegee intermediate CH2OO
Theoretical Chemistry Accounts (2022)
-
Infrared characterization of formation and resonance stabilization of the Criegee intermediate methyl vinyl ketone oxide
Communications Chemistry (2021)