Abstract
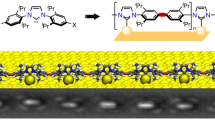
Since the first report of thiol-based self-assembled monolayers (SAMs) 30 years ago, these structures have been examined in a huge variety of applications. The oxidative and thermal instabilities of these systems are widely known, however, and are an impediment to their widespread commercial use. Here, we describe the generation of N-heterocyclic carbene (NHC)-based SAMs on gold that demonstrate considerably greater resistance to heat and chemical reagents than the thiol-based counterparts. This increased stability is related to the increased strength of the gold–carbon bond relative to that of a gold–sulfur bond, and to a different mode of bonding in the case of the carbene ligand. Once bound to gold, NHCs are not displaced by thiols or thioethers, and are stable to high temperatures, boiling water, organic solvents, pH extremes, electrochemical cycling above 0 V and 1% hydrogen peroxide. In particular, benzimidazole-derived carbenes provide films with the highest stabilities and evidence of short-range molecular ordering. Chemical derivatization can be employed to adjust the surface properties of NHC-based SAMs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
10 April 2014
In the version of this Article originally published, J. Hugh Horton should also have been denoted a corresponding author. This error has now been corrected in the online versions of the Article.
21 May 2014
In the version of this Article originally published, ref. 40 was incorrect, it should have read: Rodríguez-Castillo, M. et al. Reactivity of gold nanoparticles towards N-heterocyclic carbenes. Dalton Trans. 43, 5978–5982 (2014). This has been corrected in the online versions of the Article.
References
Nuzzo, R. G. & Allara, D. L. Adsorption of bifunctional organic disulfides on gold surfaces. J. Am. Chem. Soc. 105, 4481–4483 (1983).
Bain, C. D. et al. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J. Am. Chem. Soc. 111, 321–335 (1989).
Gates, B. D. et al. New approaches to nanofabrication: molding, printing, and other techniques. Chem. Rev. 105, 1171–1196 (2005).
Love, J. C., Estroff, L. A., Kriebel, J. K., Nuzzo, R. G. & Whitesides, G. M. Self assembled monolayers of thiolates on metals as a form of technology. Chem. Rev. 105, 1103–1169 (2005).
Drechsler, U., Erdogan, B. & Rotello, V. M. Nanoparticles, scaffolds for molecular recognition. Chem. Eur. J. 10, 5570–5579 (2004).
Jewell, A. D., Tierney, H. L. & Sykes, E. C. H. Gently lifting gold's herringbone reconstruction: trimethylphosphine on Au(111). Phys. Rev. B 82, 205401 (2010).
Leff, D. V., Brandt, L. & Heath, J. R. Synthesis and characterization of hydrophobic, organically soluble gold nanocrystals functionalized with primary amines. Langmuir 12, 4723–4730 (1996).
Gittins, D. I. & Caruso, F. Spontaneous phase transfer of nanoparticulate metals from organic to aqueous media. Angew. Chem. Int. Ed. 40, 3001–3004 (2001).
Vericat, C., Vela, M. E., Benitez, G., Carro, P. & Salvarezza, R. C. Self-assembled monolayers of thiols and dithiols on gold: new challenges for a well-known system. Chem. Soc. Rev. 39, 1805–1834 (2010).
Gooding, J. J. & Ciampi, S. The molecular level modification of surfaces: from self-assembled monolayers to complex molecular assemblies. Chem. Soc. Rev. 40, 2704–2718 (2011).
Srisombat, L., Jamison, A. C. & Lee, T. R. Stability: a key issue for self-assembled monolayers on gold as thin-film coatings and nanoparticle protectants. Colloids Surf. A 390, 1–19 (2011).
Lee, M-T., Hsueh, C-C., Freund, M. S. & Ferguson, G. S. Air oxidation of self-assembled monolayers on polycrystalline gold: the role of the gold substrate. Langmuir 14, 6419–6423 (1998).
Noh, J., Kato, H. S., Kawai, M. & Hara, M. Surface structure and interface dynamics of alkanethiol self-assembled monolayers on Au(111). J. Phys. Chem. B 110, 2793–2797 (2006).
Vericat, C., Benitez, G. A., Grumelli, D. E., Vela, M. E. & Salvarezza, R. C. Thiol-capped gold: from planar to irregular surfaces. J. Phys. Condens. Matter 20, 184004–184012 (2008).
Li, Y., Huang, J., McIver, R. T. Jr & Hemminger, J. C. Characterization of thiol self-assembled films by laser desorption Fourier transform mass spectrometry. J. Am. Chem. Soc. 114, 2428–2432 (1992).
Schoenfisch, M. H. & Pemberton, J. E. Air stability of alkanethiol self-assembled monolayers on silver and gold. J. Am. Chem. Soc. 120, 4501–4513 (1998).
Schlenoff, J. B., Li, M. & Ly, H. Stability and self-exchange in alkanethiol monolayers. J. Am. Chem. Soc. 117, 12528–12536 (1995).
Tam-Chan, S-W., Biebuyck, H. A., Whitesides, G. M., Jeon, N. & Nuzzo, R. G. Self-assembled monolayers on gold generated from alkanethiols with the structure RNHCOCH2SH. Langmuir 11, 4371–4382 (1995).
Yang, G., Amro, N. A., Starkewolfe, A. B. & Liu, G-Y. Molecular-level approach to inhibit degradations of alkanethiol self-assembled monolayers in aqueous media. Langmuir 20, 3995–4003 (2004).
Chinwangso, P., Jamison, A. C. & Lee T. R. Multidentate adsorbates for self-assembled monolayer films. Acc. Chem. Res. 44, 511–519 (2011).
Pinson, J. & Podvorica, F. Attachment of organic layers to conductive or semiconductive surfaces by reduction of diazonium salts. Chem. Soc. Rev. 34, 429–439 (2005).
Schuster, O., Yang, L., Raubenheimer, H. G. & Albrecht, M. Beyond conventional N-heterocyclic carbenes: abnormal, remote, and other classes of NHC ligands with reduced heteroatom stabilization. Chem. Rev. 109, 3445–3478 (2009).
Herrmann, W. A. N-heterocyclic carbenes: a new concept in organometallic catalysis. Angew. Chem. Int. Ed. 41, 1290–1309 (2002).
Peris, E. & Crabtree, R. H. Recent homogeneous catalytic applications of chelate and pincer N-heterocyclic carbenes. Coord. Chem. Rev. 248, 2239–2246 (2004).
Lin, J. C. Y. et al. Coinage metal N-heterocyclic carbene complexes. Chem. Rev. 109, 3561–3598 (2009).
Vougioukalakis, G. C. & Grubbs, R. H. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 110, 1746–1787 (2010).
Kantchev, E. A. B., O'Brien, C. J. & Organ, M. G. Catalysts for cross-coupling reactions – the synthetic chemist's perspective. Angew. Chem. Int. Ed. 46, 2768–2813 (2007).
Igau, A., Grützmacher, H., Baceiredo, A. & Bertrand, G. Analogous α,α′ bis carbenoid triply bonded species: synthesis of a stable λ3-phosphinocarbene-λ5 phosphaacetylene. J. Am. Chem. Soc. 110, 6463–6466 (1988).
Arduengo, A. J., Harlow, R. L. & Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 113, 361–363 (1991).
Niehues, M. et al. Synthesis and structural features of arduengo carbene complexes of group 4 metallocene cations. Organometallics 21, 2905–2911 (2002).
Pyykkö, P. & Runeberg, N. Comparative theoretical study of N-heterocyclic carbenes and other ligands bound to Au. Chem. Asian J. 1, 623–628 (2006).
Mercs, L. & Albrecht, M. Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal, luminescent, and functional materials applications. Chem. Soc. Rev. 39, 1903–1912 (2010).
Zahidi, E. M., Oudghiri-Hassani, H. & McBreen, P. H. Formation of thermally stable alkylidene layers on a catalytically active surface. Nature 409, 1023–1026 (2001).
Tulevski, G. S., Myers, M. B., Hybertsen, M. S., Steigerwald, M. L. & Nuckolls, C. Formation of catalytic metal molecule contacts. Science 309, 591–594 (2005).
Vignolle, J. & Tilley, T. D. N-heterocyclic carbene-stabilized gold nanoparticles and their assembly into 3D superlattices. Chem. Commun. 7230–7232 (2009).
Hurst, E. C., Wilson, K., Fairlamb, I. J. S. & Chechik, V. N-heterocyclic carbene coated metal nanoparticles. New J. Chem. 33, 1837–1840 (2009).
Huang, R. T. W., Wang, W. C., Yang, R. Y., Lu, J. T. & Lin, I. J. B. Liquid crystals of gold(I) N-heterocyclic carbene complexes. Dalton Trans. 38, 7121–7131 (2009).
Serpell, C. J., Cookson, J., Thompson, A. L., Brown, C.M. & Beer, P. D. Haloaurate and halopalladate imidazolium salts: structures, properties, and use as precursors for catalytic metal nanoparticles. Dalton Trans. 42, 1385–1393 (2013).
Ling, X., Schaeffer, N., Roland, S. & Pileni, M-P. Nanocrystals: why do silver and gold N-heterocyclic carbene precursors behave differently? Langmuir 29, 12647–12656 (2013).
Rodríguez-Castillo, M. et al. Reactivity of gold nanoparticles towards N-heterocyclic carbenes. Dalton Trans. 43, 5978–5982 (2014).
Zhukhovitskiy, A. V., Mavros, M. G., Van Voorhis, T. & Johnson, J. A. Addressable carbene anchors for gold surfaces. J. Am. Chem. Soc. 135, 7418–7421 (2013).
Weidner T. et al. NHC-based self-assembled monolayers on solid gold substrates. Aust. J. Chem. 64, 1177–1179 (2011).
Häkkinen, H. The gold–sulfur interface at the nanoscale. Nature Chem. 4, 443–455 (2012).
Lavrich, D. J., Wetterer, S. M., Bernasek, S. L. & Scoles, G. Physisorption and chemisorption of alkanethiols and alkyl sulfides on Au(111). J. Phys. Chem. B 102, 3456–3465 (1998).
Nuzzo, R. G., Dubois, L. H. & Allara, D. L. Fundamental studies of microscopic wetting on organic surfaces. 1. Formation and structural characterization of a self-consistent series of polyfunctional organic monolayers. J. Am. Chem. Soc. 112, 558–569 (1990).
Xu, X. et al. Abnormal N-heterocyclic carbene gold(I) complexes: synthesis, structure, and catalysis in hydration of alkynes. Organometallics 32, 164–171 (2013).
Schonenberger, C., Sondag-Huethorst, J. A. M., Jorritsma, J. & Fokkink, L. G. J. What are the ‘holes’ in self-assembled monolayers of alkanethiols on gold? Langmuir 10, 611–614 (1994).
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Prins, R., Korswagen A. R. & Kortbeek, A. G. T. G. Decomposition of the ferricenium cation by nucleophilic reagents. J. Organomet. Chem. 39, 335–344 (1972).
Pensa, E. et al. New insights into the electrochemical desorption of alkanethiol SAMs on gold Phys. Chem. Chem. Phys. 14, 12355–12367 (2012).
Acknowledgements
C.M.C., J.H.H., A.B.M., N.J.M., G.W. and H-B.K. acknowledge the Natural Sciences and Engineering Research Council of Canada (NSERC) for funding in terms of Discovery and Research Tools and Instruments grants. C.M.C. acknowledges NSERC for Discovery Accelerator Supplements funding. C.M.C., J.H.H., A.B.M. and G.W. acknowledge the Canada Foundation for Innovation for infrastructure funding. E.C.K. acknowledges NSERC for a Postgraduate Scholarship (Doctoral) fellowship. J.D.L. and A.R-W. acknowledge NSERC for Undergraduate Student Research Awards funding. E.C.K. and T.S. acknowledge the Queen's Chemistry Department for Queen's Graduate Awards. P. McBreen, K. Itami and H-P. Loock are thanked for useful discussions.
Author information
Authors and Affiliations
Contributions
C.M.C. conceived the concept, and J.H.H. and C.M.C. equally designed the experiments and prepared the manuscript using feedback from the other authors. Synthetic studies were carried out in C.M.C.'s lab, and surface studies in J.H.H.'s lab. A.B.M. and B.D. carried out STM studies, G.W. carried out solid-state MAS NMR studies, N.J.M. carried out computational studies, I.I.E., E.C.K. and T.S. worked on the syntheses of NHCs 6 and 7. O.V.Z., J.D.L. and A.R-W. worked on the synthesis and adsorption of carbenes 1–5 on surfaces. O.V.Z. and I.I.E. carried out the stability studies. I.I.E. carried out surface characterizations. Z.S. and H.B.K. carried out electrochemical measurements.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 6451 kb)
Rights and permissions
About this article
Cite this article
Crudden, C., Horton, J., Ebralidze, I. et al. Ultra stable self-assembled monolayers of N-heterocyclic carbenes on gold. Nature Chem 6, 409–414 (2014). https://doi.org/10.1038/nchem.1891
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1891
This article is cited by
-
Metal–ligand interfaces for well-defined gold nanoclusters
Science China Chemistry (2024)
-
Composite Nanoarchitectonics Towards Method for Everything in Materials Science
Journal of Inorganic and Organometallic Polymers and Materials (2024)
-
On-surface synthesis of ballbot-type N-heterocyclic carbene polymers
Nature Chemistry (2023)
-
Controlled cluster compositions
Nature Synthesis (2023)
-
N-Heterocyclic carbene-based C-centered Au(I)-Ag(I) clusters with intense phosphorescence and organelle-selective translocation in cells
Nature Communications (2022)