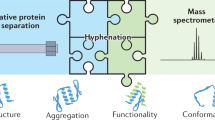
Abstract
Mass spectrometry is a vital tool for molecular characterization, and the allied technique of ion mobility is enhancing many areas of (bio)chemical analysis. Strong synergy arises between these two techniques because of their ability to ascertain complementary information about gas-phase ions. Ion mobility separates ions (from small molecules up to megadalton protein complexes) based on their differential mobility through a buffer gas. Ion mobility-mass spectrometry (IM-MS) can thus act as a tool to separate complex mixtures, to resolve ions that may be indistinguishable by mass spectrometry alone, or to determine structural information (for example rotationally averaged cross-sectional area), complementary to more traditional structural approaches. Finally, IM-MS can be used to gain insights into the conformational dynamics of a system, offering a unique means of characterizing flexibility and folding mechanisms. This Review critically describes how IM-MS has been used to enhance various areas of chemical and biophysical analysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
D'Agostino, P. A. & Chenier, C. L. Desorption electrospray ionization mass spectrometric analysis of organophosphorus chemical warfare agents using ion mobility and tandem mass spectrometry. Rapid Commun. Mass Spectrom. 24, 1617–1624 (2010).
McDaniel, E. W., Martin, D. W. & Barnes, W. S. Drift-tube mass spectrometer for studies of low-energy ion-molecule reactions. Rev. Sci. Instrum. 33, 2–7 (1962).
Mason, E. A. & Schamp, H. W. Jr. Mobility of gaseous ions in weak electric fields. Ann. Phys. 4, 233–270 (1958).
Creaser, C. S. et al. Ion mobility spectrometry: a review. Part 1. Structural analysis by mobility measurement. Analyst 129, 984–994 (2004).
Wyttenbach, T., Kemper, P. R. & Bowers, M. T. Design of a new electrospray ion mobility mass spectrometer. Int. J. Mass Spectrom. 212, 13–23 (2001).
Harvey, S. R., MacPhee, C. E. & Barran, P. E. Ion mobility mass spectrometry for peptide analysis. Methods 54, 454–461 (2011).
Giles, K. et al. Applications of a travelling-wave based radio-frequency-only stacked ring ion guide. Rapid Commun. Mass Spectrom. 18, 2401–2414 (2004).
Bush, M. F. et al. Collision cross sections of proteins and their complexes: a calibration framework and database for gas-phase structural biology. Anal. Chem. 82, 9557–9565 (2010).
Campuzano, I. et al. Structural characterization of drug-like compounds by ion mobility mass spectrometry: Comparison of theoretical and experimentally derived nitrogen collision cross sections. Anal. Chem. 84, 1026–1033 (2012).
Bush, M. F., Campuzano, I. D. & Robinson, C. V. Ion mobility mass spectrometry of peptide ions: Effects of drift gas and calibration strategies. Anal. Chem. 84, 7124–7130 (2012).
Chawner, R. et al. QconCAT standard for calibration of ion mobility-mass spectrometry systems. J. Proteome Res. 11, 5564–5572 (2012).
Purves, R. W. & Guevremont, R. Electrospray ionization high-field asymmetric waveform ion mobility spectrometry-mass spectrometry. Anal. Chem. 71, 2346–2357 (1999).
Canterbury, J. D., Yi, X., Hoopman, M. R. & MacCoss, M. J. Assessing the dynamic range and peak capacity of nanoflow LC-FAIMS-MS on an ion trap mass spectrometer for proteomics. Anal. Chem. 80, 6888–6897 (2008).
Shvartsburg, A. A., Danielson, W. F. & Smith, R. D. High-resolution differential ion mobility separations using helium-rich gases. Anal. Chem. 82, 2456–2462 (2010).
Shvartsburg, A. A. & Smith, R. D. Accelerated high-resolution differential ion mobility separations using hydrogen. Anal. Chem. 83, 9159–9166 (2011).
Shvartsburg, A. A. et al. High-definition differential ion mobility spectrometry with resolving power up to 500. J. Am. Soc. Mass Spectrom. 24, 109–114 (2013).
Knutson, E. O. & Whitby, K. T. Aerosol classification by electric mobility: Apparatus, theory, and applications. J. Aerosol Sci. 6, 443–451 (1975).
Pomareda, V., Lopez-Vidal, S., Calvo, D., Pardo, A. & Marco, S. A novel differential mobility analyzer as a VOC detector and multivariate techniques for identification and quantification. Analyst 138, 3512–3521 (2013).
de la Mora, J. F., de Juan, L., Eichler, T. & Rosell, J. Differential mobility analysis of molecular ions and nanometer particles. TRAC Trend. Anal. Chem. 17, 328–339 (1998).
Rus, J. et al. IMS-MS studies based on coupling a differential mobility analyzer (DMA) to commercial API-MS systems. Int. J. Mass Spectrom. 298, 30–40 (2010).
Creese, A. J. & Cooper, H. J. Separation and identification of isomeric glycopeptides by high field asymmetric waveform ion mobility spectrometry. Anal. Chem. 84, 2597–2601 (2012).
Varesio, E., Le Blanc, J. C. Y. & Hopfgartner, G. Real-time 2D separation by LC x differential ion mobility hyphenated to mass spectrometry. Anal. Bioanal. Chem. 402, 2555–2564 (2012).
Howdle, M. D., Eckers, C., Laures, A. M-F. & Creaser, C. S. The effect of drift gas on the separation of active pharmaceutical ingredients and impurities by ion mobility-mass spectrometry. Int. J. Mass Spectrom. 298, 72–77 (2010).
Fernández-Maestre, R., Wu, C. & Hill, H. H. Jr. Using a buffer gas modifier to change separation selectivity in ion mobility spectrometry. Int. J. Mass Spectrom. 298, 2–9 (2010).
Fernández-Maestre, R., Wu, C. & Hill, H. H. Jr. Buffer gas modifiers effect resolution in ion mobility spectrometry through selective ion-molecule clustering reactions. Rapid Commun. Mass Spectrom. 26, 2211–2223 (2012).
Holness, H. K., Jamal, A., Mebel, A. & Almirall, J. R. Separation mechanism of chiral impurities, ephedrine and pseudoephedrin, found in amphetamine-type substances using achiral modifiers in the gas phase. Anal. Bioanal. Chem. 404, 2407–2416 (2012).
Howdle, M. D., Eckers, C., Laures, A. M-F. & Creaser, C. S. The use of shift reagents in ion mobility-mass spectrometry: Studies on the complexation of an active pharmaceutical ingredient with polyethylene glycol excipients. J. Am. Soc. Mass Spectrom. 20 (2009).
Hilderbrand, A. E., Myung, S. & Clemmer, D. E. Exploring crown ethers as shift reagents for ion mobility spectrometry. Anal. Chem. 78, 6792–6800 (2006).
Bohrer, B. C. & Clemmer, D. E. Shift reagents for multidimensional ion mobility spectrometry-mass spectrometry analysis of complex peptide mixtures: evaluation of 18-Crown-6 Ether Complexes. Anal. Chem. 83, 5377–5385 (2011).
Kiss, A. & Heeren, R. M. A. Size, weight and position: ion mobility spectrometry and imaging MS combined. Anal. Bioanal. Chem. 399, 2623–2634 (2011).
Stauber, J. et al. On-tissue protein identification and imaging by MALDI-ion mobility mass spectrometry. J. Am. Soc. Mass Spectrom. 21, 338–347 (2010).
Trim, P. J. et al. Matrix-assisted laser desorption/ionization-ion mobility separation-mass spectrometry imaging of vinblastine in whole body tissue sections. Anal. Chem. 80, 8628–8634 (2008).
Shliaha, P. V., Bond, N. J., Gatto, L. & Lilley, K. S. Effects of travelling wave ion mobility separation on data independent acquisition in proteomics studies. J. Proteome Res. 12, 2323–2339 (2013).
Rodriguez-Suarez, E. et al. An ion mobility assisted data independent LC-MS strategy for the analysis of complex biological samples. Curr. Anal. Chem. 9, 199–211 (2013).
Parson, W. B. et al. Rapid analysis of isomeric exogenous metabolites by differential mobility spectrometry-mass spectrometry. Rapid Commun. Mass Spectrom. 25, 3382–3386 (2011).
Esquenazi, E., Daly, M., Bahrainwala, T., Gerwick, W. H. & Dorrestein, P. C. Ion mobility mass spectrometry enables the efficient detection and identification of halogenated natural products from cyanobacteria with minimal sample preparation. Bioorgan. Med. Chem. 19, 6639–6644 (2011).
Harry, E. L., Weston, D. J., Bristow, A. W. T., Wilson, I. D. & Creaser, C. S. An approach to enhancing coverage of the urinary metabonome using liquid chromatography-ion mobility-mass spectrometry. J. Chromatogr. B 871, 357–361 (2008).
Picotti, P., Bodenmiller, B., Mueller, L. N., Domon, B. & Aebersold, R. Full dynamic range proteome analysis of S. cerevisiae by targeted proteomics. Cell 138, 795–806 (2009).
Brownridge, P. et al. Global absolute quantification of a proteome: Challenges in the deployment of a QconCAT strategy. Proteomics 2011, 2957–2970 (2011).
Saba, J., Bonneil, E., Pomiès, C., Eng, K. & Thibault, P. Enhanced sensitivity in proteomics experiments using FAIMS coupled with a hybrid linear ion trap/orbitrap mass spectrometer. J. Proteome Res. 8, 3355–3366 (2009).
Bridon, G., Bonneil, E., Muratore-Schroeder, Caron-Lizotte, O. & Thibault, P. Improvement of phosphoproteomic analysis using FAIMS and decision tree fragmentation. Application to the insulin signaling pathway in Drosophila melanogaster S2 cells. J. Proteome Res. 11, 927–940 (2012).
Ibrahim, Y. M., Shvartsburg, A. A., Smith, R. D. & Belov, M. E. Ultrasensitive identification of localization variants of modified peptides using ion mobility spectrometry. Anal. Chem. 83, 5617–5623 (2011).
Cuyckens, F. et al. Identifying metabolite ions of peptide drugs in the presence of an in vivo matrix background. Bioanalysis 4, 595–604 (2012).
Blackburn, M. A. et al. Identification and subsequent removal of an interference by FAIMS in the bioanalysis of dianicline in animal plasma. Bioanalysis 3, 2119–2127 (2011).
Guddat, S., Thevis, M., Kapron, J., Thomas, A. & Schänzer, W. Application of FAIMS to anabolic androgenic steroids in sport drug testing. Drug Testing Anal. 1, 545–553 (2009).
Scarff, C. A., Thalassinos, K., Hilton, G. R. & Scrivens, J. H. Travelling wave ion mobility mass spectrometry studies of protein structure: Biological significance and comparison with X-ray crystallography and nuclear magnetic resonance spectroscopy measurements. Rapid Commun. Mass Spectrom. 22, 3297–3304 (2008).
Gidden, J., Bushnell, J. E. & Bowers, M. T. Gas-phase conformations and folding energetics of oligonucleotides: dTG− and dGT−. J. Am. Chem. Soc. 123, 5610–5611 (2001).
Gidden, J. & Bowers, M. T. Gas-phase conformational and energetic properties of deprotonated dinucleotides. Eur. Phys. J. D 20, 409–419 (2002).
Wyttenbach, T., Grabenauer, M., Thalassinos, K., Scrivens, J. H. & Bowers, M. T. The effect of calcium ions and peptide ligands on the relative stabilities of the calmodulin dumbbell and compact structures. J. Phys. Chem. B 114, 437–447 (2010).
Jenner, M. et al. Detection of a protein conformational equilibrium by electrospray ionisation-ion mobility-mass spectrometry. Angew. Chem. Int. Ed. 50, 8291–8294 (2011).
Bereszczak, J. Z. et al. Structure, stability and dynamics of norovirus P domain derived protein complexes studied by native mass spectrometry. J. Struct. Biol. 177, 273–282 (2012).
Shi, H., Pierson, N. A., Valentine, S. J. & Clemmer, D. E. Conformation types of ubiquitin [M+8H]8+ ions from water:methanol solutions: Evidence for the N and A states in aqueous solution. J. Phys. Chem. B 116, 3344–3352 (2012).
Smith, D. P., Giles, K., Bateman, R. H., Radford, S. E. & Ashcroft, A. E. Monitoring copopulated conformational states during protein folding events using electrospray ionization-ion mobility spectrometry-mass spectrometry. J. Am. Soc. Mass Spectrom. 18, 2180–2190 (2007).
Nabuchi, Y., Hirose, K. & Takayama, M. Ion mobility and collision-induced dissociation analysis of carbonic anhydrase 2. Anal. Chem. 82, 8890–8896 (2010).
Hyung, S-J., Robinson, C. V. & Ruotolo, B. T. Gas-phase unfolding and disassembly reveals stability differences in ligand-bound multiprotein complexes. Chem. Biol. 16, 382–390 (2009).
Hopper, J. T. S. & Oldham, N. J. Collision induced unfolding of protein ions in the gas phase studied by ion mobility-mass spectrometry: The effect of ligand binding on conformational stability. J. Am. Soc. Mass Spectrom. 20, 1851–1858 (2009).
Smith, D. P., Radford, S. E. & Ashcroft, A. E. Elongated oligomers in β2-microglobulin amyloid assembly revealed by ion mobility spectrometry-mass spectrometry. Proc. Natl Acad. Sci. USA 107, 6794–6798 (2010).
Beveridge, R., Chappuis, Q., MacPhee, C. & Barran, P. Mass spectrometry methods for intrinsically disordered proteins. Analyst 138, 32–42 (2013).
Uetrecht, C. et al. Stability and shape of hepatitis B virus capsids in vacuo. Angew. Chem. Int. Ed. 47, 6247–6251 (2008).
Ruotolo, B. T. et al. Evidence for macromolecular protein rings in the absence of bulk water. Science 310, 1658–1661 (2005).
Jurneczko, E., Kalapothakis, J., Campuzano, I. D. G., Morris, M. & Barran, P. E. Effects of drift gas on collision cross sections of a protein standard in linear drift tube and traveling wave ion mobility mass spectrometry. Anal. Chem. 84, 8524–8531 (2012).
Wyttenbach, T., von Helden, G. & Bowers, M. T. Gas-phase conformation of biological molecules: Bradykinin. J. Am. Chem. Soc. 118, 8355–8364 (1996).
Baumketner, A. et al. Amyloid β-protein monomer structure: A computational and experimental study. Protein Sci. 15, 420–428 (2006).
Dear, G. J. et al. Sites of metabolic substitution: Investigating metabolite structures utilising ion mobility and molecular modelling. Rapid Commun. Mass Spectrom. 24, 3157–3162 (2010).
Cuyckens, F. et al. Product ion mobility as a promising tool for assignment of positional isomers of drug metabolites. Rapid Commun. Mass Spectrom. 25, 3497–3503 (2011).
Wyttenbach, T., von Helden, G., Batka, J. J., Carlat, D. & Bowers, M. T. Effect of the long-range potential on ion mobility measurements. J. Am. Soc. Mass Spectrom. 8, 275–282 (1997).
Shvartsburg, A. A. & Jarrold, M. F. An exact hard-spheres scattering model for the mobilities of polyatomic ions. Chem. Phys. Lett. 261, 86–91 (1996).
Shvartsburg, A. A., Schatz, G. C. & Jarrold, M. F. Mobilities of carbon cluster ions: Critical importance of the molecular attractive potential. J. Chem. Phys. 108, 2416–2423 (1998).
Jurneczko, E. & Barran, P. E. How useful is ion mobility mass spectrometry for structural biology? The relationship between protein crystal structures and their collision cross sections in the gas phase. Analyst 136, 20–28 (2011).
Bleiholder, C., Wyttenbach, T. & Bowers, M. T. A novel projection approximation algorithm for the fast and accurate computation of molecular collision cross sections (I). Method. Int. J. Mass Spectrom. 308, 1–10 (2011).
Bleiholder, C., Contreras, S., Do, T. D. & Bowers, M. T. A novel projection approximation algorithm for the fast and accurate computation of molecular collision cross sections (II). Model parameterization and definitions of empirical shape factors for proteins. Int. J. Mass Spectrom. 345–347, 89–96 (2013).
Bleiholder, C., Contreras, S. & Bowers, M. T. A novel projection approximation algorithm for the fast and accurate computation of molecular collision cross section (IV). Application to polypeptides. Int. J. Mass Spectrom. 354–355, 275–280 (2013).
Wyttenbach, T., Bleiholder, C. & Bowers, M. T. Factors contributing to the collision cross section of polyatomic ions in the kilodalton to gigadalton range: Application to ion mobility measurements. Anal. Chem. 85, 2191–2199 (2013).
Hall, Z. & Robinson, C. V. Do charge state signatures guarantee protein conformations? J. Am. Soc. Mass Spectrom. 23, 1161–1168 (2012).
Berezovskaya, Y., Porrini, M. & Barran, P. E. The effect of salt on the conformations of three model proteins is revealed by variable temperature ion mobility mass spectrometry. Int. J. Mass Spectrom. 345, 8–18 (2013).
Shvartsburg, A. A. & Smith, R. D. Separation of protein conformers by differential ion mobility in hydrogen-rich gases. Anal. Chem. 85, 6967–6973 (2013).
Bernstein, S. L. et al. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer's disease. Nature Chem. 1, 326–331 (2009).
Grabenauer, M. et al. Spermine binding to Parkinson's protein α-synuclein and its disease-related A30P and A53T mutants. J. Phys. Chem. B 112, 11147–11154 (2008).
Shimizu, A., Ohe, T. & Chiba, M. A novel method for the determination of the site of glucuronidation by ion mobility spectrometry-mass spectrometry. Drug Metab. Dispos. 40, 1456–1459 (2012).
Baker, E. S. et al. Diastereomer assignment of an olefin-linked bis-paracyclophane by ion mobility mass spectrometry. J. Am. Chem. Soc. 126, 6255–6257 (2004).
Both, P. et al. Discrimination of epimeric glycans and glycopeptides using ion-mobility mass spectrometry and its potential for carbohydrate sequencing. Nature Chem. 6, 65–74 (2014).
Lee, S. et al. Extracted fragment ion mobility distributions: A new method for complex mixture analysis. Int. J. Mass Spectrom. 309, 154–160 (2012).
Florance, H. V. et al. Evidence for alpha-helices in the gas phase: A case study using melittin from honey bee venom. Analyst 136, 3446–3452 (2011).
Jarrold, M. F. Helices and sheets in vacuo. Phys. Chem. Chem. Phys. 9, 1659–1671 (2007).
Zilch, L. W., Kaleta, D. T., Kohtani, M., Krishnan, R. & Jarrold, M. F. Folding and unfolding of helix-turn-helix motifs in the gas phase. J. Am. Soc. Mass Spectrom. 18, 1239–1248 (2007).
McLean, J. R. et al. Factors that influence helical preferences for singly charged gas-phase peptide ions: The effects of multiple potential charge-carrying sites. J. Phys. Chem. B 114, 809–816 (2010).
Albrieux, F. et al. Conformation of polyalanine and polyglycine dications in the gas phase: Insight from ion mobility spectrometry and replica-exchange molecular dynamics. J. Phys. Chem. A 114, 6888–6896 (2010).
Albrieux, F. et al. Structural preferences of gas-phase M2TMP monomers upon sequence variations. J. Phys. Chem. A 115, 4711–4718 (2011).
Wu, C., Klasmeier, J. & Hill, H. H. Atmospheric pressure ion mobility spectrometry of protonated and sodiated peptides. Rapid Commun. Mass Spectrom. 13, 1138–1142 (1999).
Liu, D. F. et al. Oxytocin-receptor binding: Why divalent metals are essential. J. Am. Chem. Soc. 127, 2024–2025 (2005).
Rožman, M. & Gaskell, S. J. Non-covalent interactions of alkali metal cations with singly charged tryptic peptides. J. Mass Spectrom. 45, 1409–1415 (2010).
Chen, L., Gao, Y. Q. & Russell, D. H. How alkali metal ion binding alters the conformation preferences of gramicidin A: A molecular dynamics and ion mobility study. J. Phys. Chem. A 116, 689–696 (2012).
Garcia, I. R., Giles, K., Bateman, R. H. & Gaskell, S. J. Studies of peptide a- and b-type fragment ions using stable isotope labeling and integrated ion mobility/tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 19, 1781–1787 (2008).
Polfer, N. C., Bohrer, B. C., Plasencia, M. D., Paizs, B. & Clemmer, D. E. On the dynamics of fragment isomerization in collision-induced dissociation of peptides. J. Phys. Chem. A 112, 1286–1293 (2008).
Saminathan, I. S. et al. The extent and effects of peptide sequence scrambling via formation of macrocyclic b ions in model proteins. J. Am. Soc. Mass Spectrom. 21, 2085–2094 (2010).
Chawner, R., Gaskell, S. J. & Eyers, C. E. Proposal for a common nomenclature for peptide fragment ions generated following sequence scrambling during collision-induced dissociation. Rapid Commun. Mass Spectrom. 26, 205–206 (2012).
Moss, C. L. et al. Assigning structures to gas-phase peptide cations and cation-radicals. An infrared multiphoton dissociation, ion mobility, electron transfer, and computational study of a histidine peptide ion. J. Phys. Chem. B 116, 3445–3456 (2012).
van den Heuvel, R. H. H. & Heck, A. J. R. Native protein mass spectrometry: from intact oligomers to functional machineries. Curr. Opin. Chem. Biol. 8, 519–526 (2004).
Kaddis, C. S. & Loo, J. A. Native protein MS and ion mobility: Large flying proteins with ESI. Anal. Chem. 79, 1778–1784 (2007).
Heck, A. J. R. Native mass spectrometry: A bridge between interactomics and structural biology. Nature Methods 5, 927–933 (2008).
Kondrat, F. D. L., Kowald, G. R., Scarff, C. A., Scrivens, J. H. & Blindauer, C. A. Resolution of a paradox by native mass spectrometry: Facile occupation of all four metal binding sites in the dimeric zinc sensor SmtB. Chem. Commun. 49, 813–815 (2013).
Konijnenberg, A., Butterer, A. & Sobott, F. Native ion mobility-mass spectrometry and related methods in structural biology. BBA-Proteins Proteom. 1835, 1239–1256 (2013).
Hall, Z., Politis, A., Bush, M. F., Smith, L. J. & Robinson, C. V. Charge-state dependent compaction and dissociation of protein complexes: Insights from ion mobility and molecular dynamics. J. Am. Chem. Soc. 134, 3429–3438 (2012).
Raschke, T. M. Water structure and interactions with protein surfaces. Curr. Opin. Struc. Biol. 16, 152–159 (2006).
Wolynes, P. G. Biomolecular folding in vacuo!!!(?). Proc. Natl Acad. Sci. USA 92, 2426–2427 (1995).
Loo, J. A. et al. Electrospray ionization mass spectrometry and ion mobility analysis of the 20S proteasome complex. J. Am. Soc. Mass Spectrom. 16, 998–1008 (2005).
Sharon, M. et al. 20S proteasomes have the potential to keep substrates in store for continual degradation. J. Biol. Chem. 281, 9569–9575 (2006).
Ruotolo, B. T. & Robinson, C. V. Aspects of native proteins are retained in vacuum. Curr. Opin. Chem. Biol. 10, 402–408 (2006).
van Duijn, E., Barendregt, A., Synowsky, S., Versluis, C. & Heck, A. J. R. Chaperonin complexes monitored by ion mobility mass spectrometry. J. Am. Chem. Soc. 131, 1452 (2009).
Rodriguez-Cruz, S. E., Klassen, J. S. & Williams, E. R. Hydration of gas-phase Gramicidin S (M + 2H)2+ ions formed by electrospray: The transition from solution to gas-phase structure. J. Am. Soc. Mass Spectrom. 8, 565–568 (1997).
Woenckhaus, J., Hudgins, R. R. & Jarrold, M. F. Hydration of gas-phase proteins: A special hydration site on gas-phase BPTI. J. Am. Chem. Soc. 119, 9586–9587 (1997).
Fye, J. L., Woenckhaus, J. & Jarrold, M. F. Hydration of folded and unfolded gas-phase proteins: Saturation of cytochrome c and apomyoglobin. J. Am. Chem. Soc. 120, 1327–1328 (1998).
Rodriguez-Cruz, S. E., Klassen, J. S. & Williams, E. R. Hydration of gas-phase ions formed by electrospray ionization. J. Am. Soc. Mass Spectrom. 10, 958–968 (1999).
Jarrold, M. F. Peptides and proteins in the vapor phase. Annu. Rev. Phys. Chem. 51, 179–207 (2000).
Gao, B., Wyttenbach, T. & Bowers, M. T. Protonated arginine and protonated lysine: Hydration and its effect on the stability of salt-bridge structures. J. Phys. Chem. B 113, 9995–10000 (2009).
Kantardjieff, K. A. & Rupp, B. Matthews coefficient probabilities: Improved estimates for unit cell contents of proteins, DNA and protein-nucleic acid complex crystals. Protein Sci. 12, 1865–1871 (2003).
Hogan, C. J., Ruotolo, B. T., Robinson, C. V. & de la Mora, J. F. Tandem differential mobility analysis-mass spectrometry reveals partial gas-phase collapse of the GroEL complex. J. Phys. Chem. B 115, 3614–3621 (2011).
Breuker, K. & McLafferty, F. W. Stepwise evolution of protein native structure with electrospray into the gas phase, 10−12 to 102 s. Proc. Natl Acad. Sci. USA 105, 18145–18152 (2008).
Zhao, Q. et al. Effects of ion/ion proton transfer reactions on conformation of gas-phase cytochrome c ions. J. Am. Soc. Mass Spectrom. 21, 1208–1217 (2010).
Badman, E. R., Myung, S. & Clemmer, D. E. Evidence for unfolding and refolding of gas-phase cytochrome c ions in a Paul trap. J. Am. Soc. Mass Spectrom. 16, 1496–1497 (2005).
Wyttenbach, T. & Bowers, M. T. Structural stability from solution to the gas phase: Native solution structure of ubiquitin survives analysis in a solvent-free ion mobility-mass spectrometry environment. J. Phys. Chem. B 115, 12266–12275 (2011).
Lorenzi, M. et al. Conformational selection underlies recognition of a molybdoenzyme by its dedicated chaperone. PLoS ONE 7, e49523 (2012).
Wang, S. C. et al. Ion mobility mass spectrometry of two tetrameric membrane protein complexes reveals compact structures and differences in stability and packing. J. Am. Chem. Soc. 132, 15468–15470 (2010).
Boryski, A. J. & Robinson, C. V. The 'sticky business' of cleaning gas-phase membrane proteins: A detergent oriented perspective. Phys. Chem. Chem. Phys. 14, 14439–14449 (2012).
Leney, A. C., McMorran, L. M., Radford, S. E. & Ashcroft, A. E. Amphipathic polymers enable the study of functional membrane proteins in the gas phase. Anal. Chem. 84, 9841–9847 (2012).
Ashcroft, A. E. Mass spectrometry and the amyloid problem - How far can we go in the gas phase? J. Am. Soc. Mass Spectrom. 21, 1087–1096 (2010).
Smith, D. P., Woods, L. W., Radford, S. E. & Ashcroft, A. E. Structure and dynamics of oligomeric intermediates in β2-microglobulin self-assembly. Biophys. J. 101, 1238–1247 (2011).
Bernstein, S. L. et al. Amyloid β-protein: Monomer structure and early aggregation states of Aβ42 and is Pro19 alloform. J. Am. Chem. Soc. 127, 2075–2084 (2005).
Gessel, M. M. et al. Aβ(39–42) modulates Aβ oligomerization but not fibril formation. Biochemistry 51, 108–117 (2012).
Zheng, X. et al. Z-Phe-Ala-diazomethylketone (PADK) disrupts and remodels early oligomer states of Alzheimer diseases Aβ42 protein. J. Biol. Chem. 287, 6084–6088 (2012).
Amijee, H. et al. The N-methylated peptide SEN304 powerfully inhibits Aβ(1–42) toxicity by perturbing oligomer formation. Biochemistry 51, 8338–8352 (2012).
Hernandez, H. & Robinson, C. V. Determining the stoichiometry and interactions of macromolecular assemblies from mass spectrometry. Nature Protoc. 2, 715–726 (2007).
Zhou, M. et al. Mass spectrometry of intact V-type ATPases reveals bound lipids and the effects of nucleotide binding. Science 334, 380–385 (2011).
Baker, E. S., Dupuis, N. F. & Bowers, M. T. DNA hairpin, pseudoknot, and cruciform stability in a solvent-free environment. J. Phys. Chem. B 113, 1722–1727 (2009).
Chan, Y. T. et al. Design, synthesis, and traveling wave ion mobility mass spectrometry characterization of iron(II)- and ruthenium(II)-terpyridine metallomacrocycles. J. Am. Chem. Soc. 133, 11967–11976 (2011).
Larriba, C. & de la Mora, J. F. The gas phase structure of coulombically stretched polyethylene glycol ions. J. Phys. Chem. B 116, 593–598 (2012).
Arcella, A. et al. Structure of triplex DNA in the gas phase. J. Am. Chem. Soc. 134, 6596–6606 (2012).
Nagesh, N. & Chatterji, D. Ammonium ion at low concentration stabilizes the G-quadruplex formation by telomeric sequence. J. Biochem. Bioph. Meth. 30, 1–8 (1995).
Baker, E.S., Bernstein, S.L., Gabelica, V., De Pauw, E. & Bowers, M.T. G-quadruplexes in telomeric repeats are conserved in a solvent-free environment. Int. J. Mass Spectrom. 253, 225–237 (2006).
Rosu, F., Gabelica, V., Poncelet, H. & De Pauw, E. Tetramolecular G-quadruplex formation pathways studied by electrospray mass spectrometry. Nucleic Acids Res. 38, 5217–5225 (2010).
Wyttenbach, T., von Helden, G. & Bowers, M. T. Conformations of alkali ion cationized polyethers in the gas phase: Polyethylene glycol and bis[(benzo-15-crown-5)-15-ylmethyl] pimelate. Int. J. Mass Spectrom. Ion Processes 165–166, 377–390 (1997).
von Helden, G., Wyttenbach, T. & Bowers, M. T. Conformation of macromolecules in the gas phase: Use of matrix-assisted laser desorption methods in ion chromatography. Science 267, 1483–1485 (1995).
De Winter, J. et al. Size dependence of the folding of multiply charged sodium cationized polylactides revealed by ion mobility mass spectrometry and molecular modelling. Chem. Eur. J. 17, 9738–9745 (2011).
Alsharaeh, E. H. & El-Shall, M. S. Ion mobility study of the mechanism of the gas phase thermal polymerization of styrene and the structures of the early oligomers. Polymer 52, 5551–5559 (2011).
Ujma, J. et al. Shapes of supramolecular cages by ion mobility mass spectrometry. Chem. Commun. 48, 4423–4425 (2012).
Brocker, E. R., Anderson, S. E., Northrop, B. H., Stang, P. J. & Bowers, M. T. Structures of metallosupramolecular coordination assemblies can be obtained by ion mobility spectrometry-mass spectrometry. J. Am. Chem. Soc. 132, 13486–13494 (2010).
Lalli, P. M. et al. Resolution of isomeric multi-ruthenated porphyrins by travelling wave ion mobility mass spectrometry. Rapid Commun. Mass Spectrom. 26, 263–268 (2012).
Li, X. et al. Separation and characterization of metallosupramolecular libraries by ion mobility mass spectrometry. Anal. Chem. 83, 6667–6674 (2011).
Zhong, Y., Hyung, S.-J. & Ruotolo, B. T. Characterizing the resolution and accuracy of a second-generation traveling-wave ion mobility separator for biomolecular ions. Analyst 136, 3534–3541 (2011).
Li, H., Bendiak, B., Siems, W. F., Gang, D. R. & Hill, H. H. Jr. Carbohydrate structure characterisation by tandem ion mobility mass spectrometry (IMMS)2. Anal. Chem. 85, 2760–2769 (2013).
Baker, E. S. et al. Ion mobility spectrometry-mass spectrometry performance using electrodynamic ion funnels and elevated drift gas pressures. J. Am. Soc. Mass Spectrom. 18, 1176–1187 (2007).
Giles, K., Williams, J. P. & Campuzano, I. Enhancements in travelling wave ion mobility resolution. Rapid Commun. Mass Spectrom. 25, 1559–1566 (2011).
Merenbloom, S. I., Glaskin, R. S., Henson, Z. B. & Clemmer, D. E. High-resolution ion cyclotron mobility spectrometry. Anal. Chem. 81, 1482–1487 (2009).
Glaskin, R. S., Valentine, S. J. & Clemmer, D. E. A scanning frequency mode for ion cyclotron mobility spectrometry. Anal. Chem. 82, 8266–8271 (2010).
Glaskin, R. S., Ewing, M. A. & Clemmer, D. E. Ion trapping for ion mobility spectrometry measurements in a cyclical drift tube. Anal. Chem. 85, 7003–7008 (2013).
Shaffer, S. A. et al. A novel ion funnel for focusing ions at elevated pressure using electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 11, 1813–1817 (1997).
Kemper, P. R., Dupuis, N. F. & Bowers, M. T. A new, higher resolution, ion mobility mass spectrometer. Int. J. Mass Spectrom. 287, 46–57 (2009).
Ranjbar, B. & Gill, P. Circular dichroism techniques: Biomolecular and nanostructural analyses - A review. Chem. Biol. Drug Des. 74, 101–120 (2009).
Roberts, G. & Lian, L. Y. Protein NMR Spectroscopy: Practical Techniques and Applications (Wiley, 2011).
Murray, K. K. et al. Definitions of terms relating to mass spectrometry (IUPAC recommendations 2013). Pure Appl. Chem. 85, 1515–1609 (2013).
Acknowledgements
We acknowledge the financial support of the Biotechnology and Biological Sciences Research Council (BB/H007113/1, BB/G009058/1, BB/F00561X/1) and the European Commission's Seventh Framework Programme (FP7), which funded GlycoBioM. C.G. is supported by a BBSRC doctoral training grant. Finally, we thank P. Eyers for a critical reading of the review.
Author information
Authors and Affiliations
Contributions
F.L., S.W.H. and C.J.G. contributed equally to this paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lanucara, F., Holman, S., Gray, C. et al. The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nature Chem 6, 281–294 (2014). https://doi.org/10.1038/nchem.1889
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1889
This article is cited by
-
Determining the gas-phase structures of α-helical peptides from shape, microsolvation, and intramolecular distance data
Nature Communications (2023)
-
High-resolution separation of bioisomers using ion cloud profiling
Nature Communications (2023)
-
Liquid chromatography and differential mobility spectrometry—data-independent mass spectrometry for comprehensive multidimensional separations in metabolomics
Analytical and Bioanalytical Chemistry (2023)
-
Understanding of protomers/deprotomers by combining mass spectrometry and computation
Analytical and Bioanalytical Chemistry (2023)
-
Sizing up DNA nanostructure assembly with native mass spectrometry and ion mobility
Nature Communications (2022)