Abstract
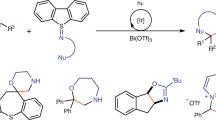
Interest in saturated N-heterocycles as scaffolds for the synthesis of bioactive molecules is increasing. Reliable and predictable synthetic methods for the preparation of these compounds, especially medium-sized rings, are limited. We describe the development of SnAP (Sn amino protocol) reagents for the transformation of aldehydes into seven-, eight- and nine-membered saturated N-heterocycles. This process occurs under mild, room-temperature conditions and offers exceptional substrate scope and functional-group tolerance. Air- and moisture-stable SnAP reagents are prepared on a multigram scale from inexpensive starting materials by simple reaction sequences. These new reagents and processes allow widely available aryl, heteroaryl and aliphatic aldehydes to be converted into diverse N-heterocycles, including diazepanes, oxazepanes, diazocanes, oxazocanes and hexahydrobenzoxazonines, by a single synthetic operation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schröter, S., Stock, C. & Bach, T. Regioselective cross-coupling reactions of multiple halogenated nitrogen-, oxygen-, and sulfur-containing heterocycles. Tetrahedron 61, 2245–2267 (2005).
Slagt, V. F., de Vries, A. H. M., de Vries, J. G. & Kellogg, R. M. Practical aspects of carbon–carbon cross-coupling reactions using heteroarenes. Org. Process Res. Dev. 14, 30–47 (2009).
Seregin, I. V. & Gevorgyan, V. Direct transition metal-catalyzed functionalization of heteroaromatic compounds. Chem. Soc. Rev. 36, 1173–1193 (2007).
Ritchie, T. J. & Macdonald, S. J. F. The impact of aromatic ring count on compound developability – are too many aromatic rings a liability in drug design? Drug Discov. Today 14, 1011–1020 (2009).
Ritchie, T. J., Macdonald, S. J. F., Young, R. J. & Pickett, S. D. The impact of aromatic ring count on compound developability: further insights by examining carbo- and hetero-aromatic and -aliphatic ring types. Drug Discov. Today 16, 164–171 (2011).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Leeson, P. D., St-Gallay, S. A. & Wenlock, M. C. Impact of ion class and time on oral drug molecular properties. Med. Chem. Comm. 2, 91–105 (2011).
Meanwell, N. A. Improving drug candidates by design: a focus on physicochemical properties as a means of improving compound disposition and safety. Chem. Res. Toxicol. 24, 1420–1456 (2011).
Vo, C-V. T., Mikutis, G. & Bode, J. W. SnAP reagents for the transformation of aldehydes into substituted thiomorpholines—an alternative to cross-coupling with saturated heterocycles. Angew. Chem. Int. Ed. 52, 1705–1708 (2013).
Galli, C. & Mandolini, L. The role of ring strain on the ease of ring closure of bifunctional chain molecules. Eur. J. Org. Chem. 3117–3125 (2000).
Mitchell, E. A., Peschiulli, A., Lefevre, N., Meerpoel, L. & Maes, B. U. W. Direct α-functionalization of saturated cyclic amines. Chem. Eur. J. 18, 10092–10142 (2012).
Müller, T. E., Hultzsch, K. C., Yus, M., Foubelo, F. & Tada, M. Hydroamination: direct addition of amines to alkenes and alkynes. Chem. Rev. 108, 3795–3892 (2008).
Hannedouche, J. & Schulz, E. Asymmetric hydroamination: a survey of the most recent developments. Chem. Eur. J. 19, 4972–4985 (2013).
Schultz, D. R. & Wolfe, J. P. Recent developments in palladium-catalyzed alkene aminoarylation reactions for the synthesis of nitrogen heterocycles. Synthesis 44, 351–361 (2012).
Zhou, Y-G. Asymmetric hydrogenation of heteroaromatic compounds. Acc. Chem. Res. 40, 1357–1366 (2007).
Campos, K. R. Direct sp3 C–H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev. 36, 1069–1084 (2007).
Coldham, I. & Leonori, D. Synthesis of 2-arylpiperidines by palladium couplings of aryl bromides with organozinc species derived from deprotonation of N-Boc-piperidine. Org. Lett. 10, 3923–3925 (2008).
Crestey, F. O., Witt, M., Jaroszewski, J. W. & Franzyk, H. Expedite protocol for construction of chiral regioselectively N-protected monosubstituted piperazine, 1,4-diazepane, and 1,4-diazocane building blocks. J. Org. Chem. 74, 5652–5655 (2009).
Deiters, A. & Martin, S. F. Synthesis of oxygen- and nitrogen-containing heterocycles by ring-closing metathesis. Chem. Rev. 104, 2199–2238 (2004).
Murakami, M., Hayashi, M. & Ito, Y. Generation of (α-aminoalkyl)samarium(III) by a new method of metalation and its carbon–carbon bond-forming reactions. Appl. Organomet. Chem. 9, 385–397 (1995).
Yoshikai, N., Mieczkowski, A., Matsumoto, A., Ilies, L. & Nakamura, E. Iron-catalyzed C–C bond formation at α-position of aliphatic amines via C–H bond activation through 1,5-hydrogen transfer. J. Am. Chem. Soc. 132, 5568–5569 (2010).
Neukom, J. D., Aquino, A. S. & Wolfe, J. P. Synthesis of saturated 1,4-benzodiazepines via Pd-catalyzed carboamination reactions. Org. Lett. 13, 2196–2199 (2011).
Hoyt, S. B. et al. Discovery of a novel class of benzazepinone Nav1.7 blockers: potential treatments for neuropathic pain. Bioorg. Med. Chem. Lett. 17, 4630–4634 (2007).
Tomaszewski, M. J. & Warkentin, J. Rate constants for aryl radical cyclization to aldimines: synthesis of tetrahydroisoquinolines by fast 6-endo closures to carbon. Tetrahedron Lett. 33, 2123–2126 (1992).
Bowman, W. R., Stephenson, P. T., Terrett, N. K. & Young, A. R. Radical cyclisations of imines and hydrazones. Tetrahedron 51, 7959–7980 (1995).
Tomaszewski, M. J., Warkentin, J. & Werstiuk, N. H. Free-radical chemistry of imines. Aust. J. Chem. 48, 291–321 (1995).
Friestad, G. K. Addition of carbon-centered radicals to imines and related compounds. Tetrahedron 57, 5461–5496 (2001).
Kim, S., Yoon, K. S. & Kim, Y. S. Kinetic studies of intramolecular additions of alkyl radicals onto imines. Tetrahedron 53, 73–80 (1997).
Acknowledgements
This work was supported by an ETH Research Grant (ETH-12 11-1) and the European Research Council (ERC Starting Grant No. 306793 – CASAA). The authors acknowledge L. Bertschi for assistance with mass spectrometry analysis.
Author information
Authors and Affiliations
Contributions
C-V.T.V. and M.U.L. performed the experiments, compound characterization and data analysis. All authors contributed to experiment design, discussions and writing the manuscripts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 7697 kb)
Supplementary information
Crystallographic data for compound 11b (CIF 362 kb)
Rights and permissions
About this article
Cite this article
Vo, CV., Luescher, M. & Bode, J. SnAP reagents for the one-step synthesis of medium-ring saturated N-heterocycles from aldehydes. Nature Chem 6, 310–314 (2014). https://doi.org/10.1038/nchem.1878
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1878
This article is cited by
-
Expedient syntheses of N-heterocycles via intermolecular amphoteric diamination of allenes
Nature Communications (2018)
-
Dehydrogenative reagent-free annulation of alkenes with diols for the synthesis of saturated O-heterocycles
Nature Communications (2018)
-
Stereoselective access to tubuphenylalanine and tubuvaline: improved Mn-mediated radical additions and assembly of a tubulysin tetrapeptide analog
The Journal of Antibiotics (2016)