Abstract
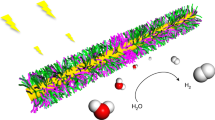
Integration into a soft material of all the molecular components necessary to generate storable fuels is an interesting target in supramolecular chemistry. The concept is inspired by the internal structure of photosynthetic organelles, such as plant chloroplasts, which colocalize molecules involved in light absorption, charge transport and catalysis to create chemical bonds using light energy. We report here on the light-driven production of hydrogen inside a hydrogel scaffold built by the supramolecular self-assembly of a perylene monoimide amphiphile. The charged ribbons formed can electrostatically attract a nickel-based catalyst, and electrolyte screening promotes gelation. We found the emergent phenomenon that screening by the catalyst or the electrolytes led to two-dimensional crystallization of the chromophore assemblies and enhanced the electronic coupling among the molecules. Photocatalytic production of hydrogen is observed in the three-dimensional environment of the hydrogel scaffold and the material is easily placed on surfaces or in the pores of solid supports.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Umena, Y., Kawakami, K., Shen, J-R. & Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–60 (2011).
Amunts, A., Drory, O. & Nelson, N. The structure of a plant photosystem I supercomplex at 3.4 Å resolution. Nature 447, 58–63 (2007).
Hull, J. F. et al. Highly active and robust Cp* iridium complexes for catalytic water oxidation. J. Am. Chem. Soc. 131, 8730–8731 (2009).
Kanan, M. W. & Nocera, D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 (2008).
Karunadasa, H. I. et al. A molecular MoS2 edge site mimic for catalytic hydrogen generation. Science 335, 698–702 (2012).
Yin, Q. et al. A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science 328, 342–345 (2010).
Helm, M. L., Stewart, M. P., Bullock, R. M., DuBois, M. R. & DuBois, D. L. A synthetic nickel electrocatalyst with a turnover frequency above 100,000 s−1 for H2 production. Science 333, 863–866 (2011).
Han, Z., Qiu, F., Eisenberg, R., Holland, P. L. & Krauss, T. D. Robust photogeneration of H2 in water using semiconductor nanocrystals and a nickel catalyst. Science 338, 1321–1324 (2012).
Boettcher, S. W. et al. Photoelectrochemical hydrogen evolution using Si microwire arrays. J. Am. Chem. Soc. 133, 1216–1219 (2011).
Veldkamp, B. S. et al. Photoinitiated multi-step charge separation and ultrafast charge transfer induced dissociation in a pyridyl-linked photosensitizer–cobaloxime assembly. Energy Environ. Sci. 6, 1917–1928 (2013).
Poddutoori, P. et al. Photoinitiated multistep charge separation in ferrocene–zinc porphyrin–diiron hydrogenase model complex triads. Energy Environ. Sci. 4, 2441–2450 (2011).
Reece, S. Y. et al. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 334, 645–648 (2011).
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
Babu, S. S., Praveen, V. K. & Ajayaghosh, A. Functional p-gelators and their applications. Chem. Rev. 114, 1973–2129 (2014).
Feiler, L., Langhals, H. & Polborn, K. Synthesis of perylene-3,4-dicarboximides – novel highly photostable fluorescent dyes. Liebigs Ann. 1229–1244 (1995).
Lefler, K. M., Co, D. T. & Wasielewski, M. R. Self-assembly-induced ultrafast photodriven charge separation in perylene-3,4-dicarboximide-based hydrogen-bonded foldamers. J. Phys. Chem. Lett. 3, 3798–3805 (2012).
Samorì, P. et al. Self-assembly of perylene monoimide substituted hexa-peri-hexabenzocoronenes: dyads and triads at surfaces. Adv. Mater. 18, 1317–1321 (2006).
Bullock, J. E. et al. Photophysics and redox properties of rylene imide and diimide dyes alkylated ortho to the imide groups. J. Phys. Chem. B 114, 1794–1802 (2010).
Cui, H. et al. Spontaneous and X-ray-triggered crystallization at long range in self-assembling filament networks. Science 327, 555–559 (2010).
Tasios, N. et al. Self-assembly, dynamics, and phase transformation kinetics of donor–acceptor substituted perylene derivatives. J. Am. Chem. Soc. 132, 7478–7487 (2010).
Shahar, C. et al. Self-assembly of light-harvesting crystalline nanosheets in aqueous media. ACS Nano 7, 3547–3556 (2013).
Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 1564–1579 (2004).
Spano, F. C. The spectral signatures of Frenkel polarons in H- and J-aggregates. Acc. Chem. Res. 43, 429–439 (2010).
Zsila, F., Bikádi, Z., Keresztes, Z., Deli, J. & Simonyi, M. Investigation of the self-organization of lutein and lutein diacetate by electronic absorption, circular dichroism spectroscopy, and atomic force microscopy. J. Phys. Chem. B 105, 9413–9421 (2001).
Spano, F. C., Meskers, S. C. J., Hennebicq, E. & Beljonne, D. Probing excitation delocalization in supramolecular chiral stacks by means of circularly polarized light: experiment and modeling. J. Am. Chem. Soc. 129, 7044–7054 (2007).
Agranovich, V. M. Excitations in Organic Solids (Oxford Univ. Press, 2009).
Okeyoshi, K. & Yoshida, R. Hydrogen generating gel systems induced by visible light. Soft Matter 5, 4118–4123 (2009).
Yuhas, B. D. et al. Biomimetic multifunctional porous chalcogels as solar fuel catalysts. J. Am. Chem. Soc. 133, 7252–7255 (2011).
Jiang, D-L. et al. Photosensitized hydrogen evolution from water using conjugated polymers wrapped in dendrimeric electrolytes. J. Am. Chem. Soc. 126, 12084–12089 (2004).
Utschig, L. M. et al. Photocatalytic hydrogen production from noncovalent biohybrid photosystem I/Pt nanoparticle complexes. J. Phys. Chem. Lett. 2, 236–241 (2011).
Wilson, A. D. et al. Nature of hydrogen interactions with Ni(II) complexes containing cyclic phosphine ligands with pendant nitrogen bases. Proc. Natl Acad. Sci. USA 104, 6951–6956 (2007).
McLaughlin, M. P., McCormick, T. M., Eisenberg, R. & Holland, P. L. A stable molecular nickel catalyst for the homogeneous photogeneration of hydrogen in aqueous solution. Chem. Commun. 47, 7989–7991 (2011).
Das, A., Han, Z., Haghighi, M. G. & Eisenberg, R. Photogeneration of hydrogen from water using CdSe nanocrystals demonstrating the importance of surface exchange. Proc. Natl Acad. Sci. USA 110, 16716–16723 (2013).
Jain, A. et al. Incorporating peptides in the outer-coordination sphere of bioinspired electrocatalysts for hydrogen production. Inorg. Chem. 50, 4073–4085 (2011).
Acknowledgements
This work was supported as part of the Argonne-Northwestern Solar Energy Research (ANSER) Center, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under Award No. DE-SC0001059. Use of the Advanced Photon Source (APS) was supported by the US Department of Energy, Office of Science, Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. SAXS experiments were performed at the DuPont–Northwestern–Dow Collaborative Access Team (DND-CAT) located at Sector 5 of APS. DND-CAT is supported by E.I. DuPont de Nemours & Co., The Dow Chemical Company and Northwestern University. WAXS experiments were conducted at BioCARS Sector 14 at the APS supported by grants from the National Center for Research Resources (5P41RR007707) and the National Institute of General Medical Sciences (8P41GM103543) from the National Institutes of Health. GIXS data were collected at Sector 12 of the APS. We thank the Biological Imaging Facility at Northwestern for the use of TEM equipment, and the Electron Probe Instrumentation Center facilities of the Northwestern University Atomic and Nanoscale Characterization Experimental Center for the use of SEM equipment. NMR and MS equipment at the Integrated Molecular Structure Education and Research Center was supported by the National Science Foundation under CHE-9871268. The authors acknowledge J. Lehrman and Y. Velichko of the Stupp laboratory and K. Lefler, W. Salamant, M. Vagnini, B. Veldkamp and D. Gardner of the Wasielewski laboratory for helpful discussions.
Author information
Authors and Affiliations
Contributions
A.S.W., R.V.K., L.C.P., M.R.W. and S.I.S designed the experiments. A.S.W., R.V.K., M.M., A.R.K., A.P.S.S. and D.J.K. performed the experimental work. A.S.W., R.V.K., L.C.P., M.R.W. and S.I.S. analysed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1958 kb)
Rights and permissions
About this article
Cite this article
Weingarten, A., Kazantsev, R., Palmer, L. et al. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nature Chem 6, 964–970 (2014). https://doi.org/10.1038/nchem.2075
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2075
This article is cited by
-
Enzymatic polymerization of enantiomeric L−3,4-dihydroxyphenylalanine into films with enhanced rigidity and stability
Nature Communications (2023)
-
Packing-induced selectivity switching in molecular nanoparticle photocatalysts for hydrogen and hydrogen peroxide production
Nature Nanotechnology (2023)
-
Floatable photocatalytic hydrogel nanocomposites for large-scale solar hydrogen production
Nature Nanotechnology (2023)
-
Photocatalytic sacrificial H2 evolution dominated by micropore-confined exciton transfer in hydrogen-bonded organic frameworks
Nature Catalysis (2023)
-
Improving hole transfer of boron nitride quantum dots modified PDI for efficient photodegradation
Frontiers of Chemical Science and Engineering (2023)