Abstract
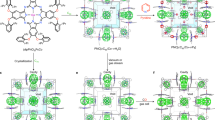
Molecular adsorption is a fundamental phenomenon in porous materials and is usually characterized by the efficiency and selectivity of molecular separations and reactions. However, for functional porous materials, analysis of the dynamic behaviour of molecular adsorbents is a major challenge. Here, we use in situ single-crystal X-ray diffraction to analyse multi-step molecular adsorption in a crystalline nanochannel of a metal-macrocycle framework. The pore surface of the metal-macrocycle framework crystal contains five different enantiomerically paired binding pockets, to which the adsorption of a (1R)-1-(3-chlorophenyl)ethanol solution was monitored with time. The resulting X-ray snapshot analyses suggest that the guest adsorption process takes a two-step pathway before equilibrium, in which the guest molecule is temporarily trapped by a neighbouring binding site. This demonstrates the potential for using X-ray analyses to visualize a transient state during a non-covalent self-assembly process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Van Bekkum, H., Flanigen, E. M., Jacobs, P. A. & Jansen, J. C. (eds) Introduction to Zeolite Science and Practice (Elsevier, 2001).
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003).
Férey, G. Hybrid porous solids: past, present, future. Chem. Soc. Rev. 37, 191–214 (2008).
Bradshaw, D., Claridge, J. B., Cussen, E. J., Prior, T. J. & Rosseinsky, M. J. Design, chirality, and flexibility in nanoporous molecule-based materials. Acc. Chem. Res. 38, 273–282 (2005).
Holst, J. R., Trewin, A. & Cooper, A. I. Porous organic molecules. Nature Chem. 2, 915–920 (2010).
Desiraju, G. R. Crystal engineering: a holistic view. Angew. Chem. Int. Ed. 46, 8342–8356 (2007).
Bezzu, C. G., Helliwell, M., Warren, J. E., Allan, D. R. & Mckeown, N. B. Heme-like coordination chemistry within nanoporous molecular crystals. Science 327, 1627–1630 (2010).
Sumida, K. et al. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 112, 724–781 (2012).
Hasell, T., Schmidtmann, M. & Cooper, A. I. Molecular doping of porous organic cages. J. Am. Chem. Soc. 133, 14920–14923 (2011).
Li, J-R., Sculley, J. & Zhou, H-C. Metal–organic frameworks for separations. Chem. Rev. 112, 869–932 (2012).
Kreno, L. E. et al. Metal–organic framework materials as chemical sensors. Chem. Rev. 112, 1105–1125 (2012).
Yoon, M., Srirambalaji, R. & Kim, K. Homochiral metal–organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 112, 1196–1231 (2012).
Matsuda, R. et al. Highly controlled acetylene accommodation in a metal–organic microporous material. Nature 436, 238–241 (2005).
Vaidhyanathan, R. et al. Direct observation and quantification of CO2 binding within an amine-functionalized nanoporous solid. Science 330, 650–653 (2010).
Jacobs, T. et al. In situ X-ray structural studies of a flexible host responding to incremental gas loading. Angew. Chem. Int. Ed. 51, 4913–4916 (2012).
Krishna, R. Diffusion in porous crystalline materials. Chem. Soc. Rev. 41, 3099–3118. (2012).
Hibbe, F. et al. The nature of surface barriers on nanoporous solids explored by microimaging of transient guest distributions. J. Am. Chem. Soc. 133, 2804–2807 (2011).
Fletcher, A. J., Cussen, E. J., Bradshaw, D., Rosseinsky, M. J. & Thomas, K. M. Adsorption of gases and vapors on nanoporous Ni2(4,4′-bipyridine)3(NO3)4 metal–organic framework materials templated with methanol and ethanol: structural effects in adsorption kinetics, J. Am. Chem. Soc. 126, 9750–9759 (2004).
Wang, C. & Lin, W. Diffusion-controlled luminescence quenching in metal–organic frameworks. J. Am. Chem. Soc. 133, 4232–4235 (2011).
Han, S., Hermans, T. M., Fuller, P. E., Wei, Y. & Grzybowski, B. A. Transport into metal–organic frameworks from solution is not purely diffusive. Angew. Chem. Int. Ed. 51, 2662–2666 (2012).
Liao, Y., Yang, S. K., Koh, K., Matzger, A. J. & Biteen, J. S. Heterogeneous single-molecule diffusion in one-, two-, and three-dimensional microporous coordination polymers: directional, trapped, and immobile guests. Nano Lett. 12, 3080–3085 (2012).
Mitra, T. et al. Molecular shape sorting using molecular organic cages. Nature Chem. 5, 276–281 (2013).
Tashiro, S., Kubota, R. & Shionoya, M. Metal-macrocycle framework (MMF): supramolecular nano-channel surfaces with shape sorting capability. J. Am. Chem. Soc. 134, 2461–2464 (2012).
Kubota, R., Tashiro, S., Umeki, T. & Shionoya, M. Non-covalent surface modification of metal-macrocycle framework with mono-substituted benzenes. Supramol. Chem. 24, 867–877 (2012).
Tashiro, S. & Shionoya, M. Cavity-assembled porous solids (CAPS) for nanospace-specific funcitons. Bull. Chem. Soc. Jpn 87, 643–654 (2014).
Tashiro, S., Umeki, T., Kubota, R. & Shionoya, M. Simultaneous arrangement of up to three different molecules on the pore surface of a metal-macrocycle framework: cooperation and competition. Angew. Chem. Int. Ed. 53, 8310–8315 (2014).
Coppens, P., Vorontsov, I. I., Graber, T., Gembicky, M. & Kovalevsky, A. Y. The structure of short-lived excited states of molecular complexes by time-resolved X-ray diffraction. Acta Crystallogr. A 61, 162–172 (2005).
Cole, J. M. Single-crystal X-ray diffraction studies of photo-induced molecular species. Chem. Soc. Rev. 33, 501–513 (2004).
Kawano, M., Kobayashi, Y., Ozeki, T. & Fujita, M. Direct crystallographic observation of a coordinatively unsaturated transition-metal complex in situ generated within a self-assembled cage. J. Am. Chem. Soc. 128, 6558–6559 (2006).
Moffat, K. Time-resolved biochemical crystallography: a mechanistic perspective. Chem. Rev. 101, 1569–1582 (2001).
Kovaleva, E. G. & Lipscomb, J. D. Crystal structures of Fe2+ dioxygenase superoxo, alkylperoxo, and bound product intermediates. Science 316, 453–457 (2007).
Kawamichi, T., Haneda, T., Kawano, M. & Fujita, M. X-ray observation of a transient hemiaminal trapped in a porous network. Nature 461, 633–635 (2009).
Acknowledgements
This research was supported in part by KAKENHI (nos. 21225003 and 23655117), the Japan Society for the Promotion of Science and MEXT, Japan. The authors acknowledge Takasago International Corporation for the provision of (1R)-1-(3-chlorophenyl)ethanol. The authors thank J. Tucker and J-L. Duprey for helpful discussions.
Author information
Authors and Affiliations
Contributions
M. Shionoya and S.T. designed the project and analysed the results. All authors prepared the manuscript. R.K. performed the experimental work. M. Shiro confirmed the validity of the X-ray crystallographic analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2175 kb)
Supplementary information
Crystallographic data for compound MMF_time1 (CIF 5595 kb)
Supplementary information
Crystallographic data for compound MMF_time2 (CIF 8022 kb)
Supplementary information
Crystallographic data for compound MMF_time3 (CIF 8013 kb)
Supplementary information
Crystallographic data for compound MMF_time4 (CIF 7958 kb)
Rights and permissions
About this article
Cite this article
Kubota, R., Tashiro, S., Shiro, M. et al. In situ X-ray snapshot analysis of transient molecular adsorption in a crystalline channel. Nature Chem 6, 913–918 (2014). https://doi.org/10.1038/nchem.2044
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2044
This article is cited by
-
Effector-dependent structural transformation of a crystalline framework with allosteric effects on molecular recognition ability
Nature Communications (2023)
-
Highly responsive nature of porous coordination polymer surfaces imaged by in situ atomic force microscopy
Nature Chemistry (2019)
-
Observation of gold sub-nanocluster nucleation within a crystalline protein cage
Nature Communications (2017)
-
Coordination templated [2+2+2] cyclotrimerization in a porous coordination framework
Nature Communications (2015)