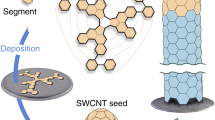
Abstract
Carbon nanotubes (CNTs), tubular molecular entities that consist of sp2-hybridized carbon atoms, are currently produced as mixtures that contain tubes of various diameters and different sidewall structures. The electronic and optical properties of CNTs are determined by their diameters and sidewall structures and so a controlled synthesis of uniform-diameter, single-chirality CNTs—a significant chemical challenge—would provide access to pure samples with predictable properties. Here we report a rational bottom-up approach to synthesize structurally uniform CNTs using carbon nanorings (cycloparaphenylenes) as templates and ethanol as the carbon source. The average diameter of the CNTs formed is close to that of the carbon nanorings used, which supports the operation of a ‘growth-from-template’ mechanism in CNT formation. This bottom-up organic chemistry approach is intrinsically different from other conventional approaches to making CNTs and, if it can be optimized sufficiently, offers a route to the programmable synthesis of structurally uniform CNTs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dresselhaus, M., Dresselhaus, G. & Avouris, P. Carbon Nanotubes: Synthesis, Properties and Applications (Springer, 2001).
Avouris, P., Chen, Z. & Perebeinos, V. Carbon-based electronics. Nature Nanotech. 2, 605–615 (2007).
Avouris, P., Freitag, M. & Perebeinos, V. Carbon-nanotube photonics and optoelectronics. Nature Photon. 2, 341–350 (2008).
Sgobba, V. & Guldi, D. M. Carbon-nanotubes electronic/electrochemical properties and application for nanoelectronics and photonics. Chem. Soc. Rev. 38, 165–184 (2009).
Thess, A. et al. Crystalline ropes of metallic carbon nanotubes. Science 273, 483–487 (1996).
Bachilo, S. M. et al. Narrow (n,m)-distribution of single-walled carbon nanotubes grown using a solid supported catalyst. J. Am. Chem. Soc. 125, 11186–11187 (2003).
Chiang, W-H. & Sankaran, R. M. Linking catalyst composition to chirality distributions of as-grown single-walled carbon nanotubes by tuning NixFe1–x nanoparticles. Nature Mater. 8, 882–886 (2009).
Kato, T. & Hatakeyama, R. Direct growth of short single-walled carbon nanotubes with narrow-chirality distribution by time-programmed plasma chemical vapor deposition. ACS Nano 4, 7395–7400 (2010).
Tu, X., Manohar, S., Jagota, A. & Zheng, M. DNA sequence motifs for structure-specific recognition and separation of carbon nanotubes. Nature 460, 250–253 (2009).
Tu, X., Walker, A. R. H., Khripin, C. Y. & Zheng, M. Evolution of DNA sequences toward recognition of metallic armchair carbon nanotubes. J. Am. Chem. Soc. 133, 12998–13001 (2011).
Arnold, M. S., Green, A. A., Hulvat, J. F., Stupp, S. I. & Hersam, M. C. Sorting carbon nanotubes by electronic structure using density differentiation. Nature Nanotech. 1, 60–65 (2006).
Ghosh, S., Bachilo, S. M. & Weisman, R. B. Advanced sorting of single-walled carbon nanotubes by nonlinear density-gradient ultracentrifugation. Nature Nanotech. 5, 443–450 (2010).
Liu, H., Nishide, D., Tanaka, T. & Kataura, H. Large-scale single-chirality separation of single-wall carbon nanotubes by simple gel chromatography. Nature Commun. 2, 309 (2011).
Fort, E. H. & Scott, L. T. Carbon nanotubes from short hydrocarbon templates. Energy analysis of the Diels–Alder cycloaddition/rearomatization growth strategy. J. Mater. Chem. 21, 1373–1381 (2011).
Scott, L. T. et al. A short, rigid, structurally pure carbon nanotube by stepwise chemical synthesis. J. Am. Chem. Soc. 134, 107–110 (2012).
Omachi, H., Segawa, Y. & Itami, K. Synthesis of cycloparaphenylenes and related carbon nanorings: a step toward the controlled synthesis of carbon nanotubes. Acc. Chem. Res. 45, 1378–1389 (2012).
Jasti, R. & Bertozzi, C. R. Progress and challenges for the bottom-up synthesis of carbon nanotubes with discrete chirality. Chem. Phys. Lett. 494, 1–7 (2010).
Yao, Y., Feng, C., Zhang, J. & Liu, Z. ‘Cloning’ of single-walled carbon nanotubes via open-end growth mechanism. Nano Lett. 9, 1673–1677 (2009).
Yu, X. et al. Cap formation engineering: from opened C60 to single-walled carbon nanotubes. Nano Lett. 10, 3343–3349 (2010).
Li, H-B., Page, A. J., Irle, S. & Morokuma, K. Single-walled carbon nanotube growth from chiral carbon nanorings: prediction of chirality and diameter influence on growth rates. J. Am. Chem. Soc. 134, 15887–15896 (2012).
Jasti, R., Bhattacharjee, J., Neaton, J. B. & Bertozzi, C. R. Synthesis, characterization, and theory of [9]-, [12]-, and [18]cycloparaphenylene: carbon nanohoop structures. J. Am. Chem. Soc. 130, 17646–17647 (2008).
Takaba, H., Omachi, H., Yamamoto, Y., Bouffard, J. & Itami, K. Selective synthesis of [12]cycloparaphenylene. Angew. Chem. Int. Ed. 48, 6112–6116 (2009).
Yamago, S., Watanabe, Y. & Iwamoto, T. Synthesis of [8]cycloparaphenylene from a square-shaped tetranuclear platinum complex. Angew. Chem. Int. Ed. 49, 757–759 (2010).
Omachi, H., Matsuura, S., Segawa, Y. & Itami, K. A modular and size-selective synthesis of [n]cycloparaphenylenes: a step toward the selective synthesis of [n,n]single-walled carbon nanotubes. Angew. Chem. Int. Ed. 49, 10202–10205 (2010).
Segawa, Y. et al. Concise synthesis and crystal structure of [12]cycloparaphenylene. Angew. Chem. Int. Ed. 50, 3244–3248 (2011).
Iwamoto, T., Watanabe, Y., Sakamoto, Y., Suzuki, T. & Yamago, S. Selective and random syntheses of [n]cycloparaphenylenes (n = 8–13) and size dependence of their electronic properties. J. Am. Chem. Soc. 133, 8354–8361 (2011).
Sisto, T. J., Golder, M. R., Hirst, E. S. & Jasti, R. Selective synthesis of strained [7]cycloparaphenylene: an orange-emitting fluorophore. J. Am. Chem. Soc. 133, 15800–15802 (2011).
Xia, J. & Jasti, R. Synthesis, characterization, and crystal structure of [6]cycloparaphenylene. Angew. Chem. Int. Ed. 51, 2474–2476 (2012).
Ishii, Y. et al. Size-selective synthesis of [9]–[11] and [13]cycloparaphenylenes. Chem. Sci. 3, 2340–2345 (2012).
Omachi, H., Segawa, Y. & Itami, K. Synthesis and racemization process of chiral carbon nanorings: a step toward the chemical synthesis of chiral carbon nanotubes. Org. Lett. 13, 2480–2483 (2011).
Yagi, A., Segawa, Y. & Itami, K. Synthesis and properties of [9]cyclo-1,4-naphthylene: a π-extended carbon nanoring. J. Am. Chem. Soc. 134, 2962–2965 (2012).
Matsui, K., Segawa, Y. & Itami, K. Synthesis and properties of cycloparaphenylene-2,5-pyridylidene: a nitrogen-containing carbon nanoring. Org. Lett. 14, 1888–1891 (2012).
Hitosugi, S., Nakanishi, W., Yamasaki, T. & Isobe, H. Bottom-up synthesis of finite models of helical (n,m)-single-wall carbon nanotubes. Nature Commun. 2, 492 (2011).
Hitosugi, S., Yamasaki, T. & Isobe, H. Bottom-up synthesis and thread-in-bead structures of finite (n,0)-zigzag single-wall carbon nanotubes. J. Am. Chem. Soc. 134, 12442–12445 (2012).
Jorio, A. et al. Structural (n,m) determination of isolated single-wall carbon nanotubes by resonant Raman scattering. Phys. Rev. Lett. 86, 1118–1121 (2001).
Kataura, H. et al. Optical properties of single-wall carbon nanotubes. Synth. Metals 103, 2555–2558 (1999).
Fort, E. H., Jeffreys, M. S. & Scott, L. T. Diels–Alder cycloaddition of acetylene gas to a polycyclic aromatic hydrocarbon bay region. Chem. Commun. 48, 8102–8104 (2012).
Luo, Y-R. Handbook of Bond Dissociation Energies in Organic Compounds (CRC, 2003).
Acknowledgements
This work was supported by the Funding Program for Next Generation World-Leading Researchers from the Japan Society for the Promotion of Science (JSPS) (220GR049 to K.I.) and the Tokuyama Science Foundation. FUJIFILM Corporation is acknowledged for various types of support. H.O. thanks JSPS for a predoctoral fellowship. We are very grateful to H. Shinohara and R. Kitaura (Nagoya University) for providing access to their instruments as well as for discussion. C. M. Crudden (Queen's University, Canada) is acknowledged for comments and discussion. We thank T. Kitagawa, K. Miyaura and S. Naruse (Nagoya University) for their technical assistance.
Author information
Authors and Affiliations
Contributions
H.O. synthesized [9]CPP and [12]CPP. H.O. and T.N. performed the CNT growth experiments and analyses. E.T. conducted the CPP decomposition experiments. Y.S. provided critical advice. K.I. conceived the concept and prepared the manuscript with feedback from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 7253 kb)
Rights and permissions
About this article
Cite this article
Omachi, H., Nakayama, T., Takahashi, E. et al. Initiation of carbon nanotube growth by well-defined carbon nanorings. Nature Chem 5, 572–576 (2013). https://doi.org/10.1038/nchem.1655
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1655
This article is cited by
-
Carbon nanotubes and nanobelts as potential materials for biosensor
Scientific Reports (2023)
-
Carbon Nanotube (10, 0) and Silicon Nanotube (7, 0) as a Novel Material for Drug Delivery of Substituted Eugenols as Antioxidant Drugs
Silicon (2023)
-
Synthesis of an all-carbon conjugated polymeric segment of carbon nanotubes and its application for lithium-ion batteries
Nano Research (2023)
-
Synergistic modulation of spin and fluorescence signals in a nano-Saturn assembled by a metallofullerene and cycloparaphenylene nanohoop
Nano Research (2023)
-
Functionalized multi-walled carbon nanotubes for oil spill cleanup from water
Clean Technologies and Environmental Policy (2022)