Abstract
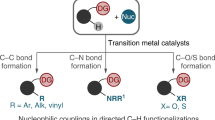
The beginning of the twenty-first century has witnessed significant advances in the field of C–H bond activation, and this transformation is now an established piece in the synthetic chemists' toolbox. This methodology has the potential to be used in many different areas of chemistry, for example it provides a perfect opportunity for the late-stage diversification of various kinds of organic scaffolds, ranging from relatively small molecules like drug candidates, to complex polydisperse organic compounds such as polymers. In this way, C–H activation approaches enable relatively straightforward access to a plethora of analogues or can help to streamline the lead-optimization phase. Furthermore, synthetic pathways for the construction of complex organic materials can now be designed that are more atom- and step-economical than previous methods and, in some cases, can be based on synthetic disconnections that are just not possible without C–H activation. This Perspective highlights the potential of metal-catalysed C–H bond activation reactions, which now extend beyond the field of traditional synthetic organic chemistry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Godula, K. & Sames, D. C–H bond functionalization in complex organic synthesis. Science 312, 67–72 (2006).
Crabtree, R. H. Alkane C–H activation and functionalization with homogenous transition metal catalysts: a century of progress-a new millennium in prospect. J. Chem. Soc. Dalton Trans. 2437–2450 (2001).
Ackermann, L. Carboxylate-assisted transition-metal-catalyzed C–H bond functionalization: mechanism and scope. Chem. Rev. 111, 1315–1345 (2011).
Yeung, C. S. & Dong, V. M. Catalytic dehydrogenative cross-coupling: forming carbon–carbon bonds by oxidizing two carbon–hydrogen bonds. Chem. Rev. 111, 1215–1292 (2011).
Wencel-Delord, J., Dröge, T., Liu, F. & Glorius, F. Towards mild metal-catalyzed C–H bond activation. Chem. Soc. Rev. 40, 4740–4761 (2011).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Arockiam, P. B., Bruneau, C. & Dixneuf, P. H. Ruthenium(II)-catalyzed C–H bond activation and functionalization. Chem. Rev. 112, 5879–5918 (2012).
Colby, D. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 110, 624–655 (2010).
Chen, X., Engle, K. M., Wang, D.-H. & Yu, J.-Q. Palladium(II)-catalyzed C–H activation/C–C cross-coupling reactions: Versatility and practicality. Angew. Chem. Int. Ed. 48, 5094–5115 (2009).
Li, B.-J. & Shi, Z.-J. From C(sp2)–H to C(sp3)–H: systematic studies on transition metal-catalyzed oxidative C–C formation. Chem. Soc. Rev. 41, 5588–5598 (2012).
Yamaguchi, J., Yamaguchi, A. D. & Itami, K. C–H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012).
Chen, D. Y.-K. & Youn, S. W. C–H Activation: A complementary tool in the total synthesis of complex natural products. Chem. Eur. J. 18, 9452–9474 (2012).
McMurray, L., O'Hara, F. & Gaunt, M. J. Recent developments in natural product synthesis using metal-catalyzed C–H bond functionalisation. Chem. Soc. Rev. 40, 1885–1898 (2011).
Dai, H.-X., Stepan, A. F., Plummer, M. S., Zhang, Y.-H. & Yu, J.-Q. Divergent C–H functionalizations directed by sulfonamide pharmacophores: Late-stage diversification as a tool for drug discovery. J. Am. Chem. Soc. 133, 7222–7228 (2011).
Pham, M. V., Ye, B. & Cramer, N. Access to sultams by rhodium(III)-catalyzed directed C–H activation. Angew. Chem. Int. Ed. 51, 10610–10614 (2012).
Meyer, C. et al. Exploitation of an additional hydrophobic pocket of σ1 receptors: Late-stage diverse modifications of spirocyclic thiophenes by C–H bond functionalization. Org. Biomol. Chem. 9, 8016–8029 (2011).
Alberico, D., Scott, M. E. & Lautens, M. Aryl–aryl bond formation by transition-metal-catalyzed direct arylation. Chem. Rev. 107, 174–238 (2007).
Roger, J., Gottumukkala, A. L. & Doucet, H. Palladium-catalyzed C3 or C4 direct arylation of heteroaromatic compounds with aryl halides by C–H bond activation. ChemCatChem 2, 20–40 (2010).
Meyer, C. et al. Late-stage C–H bond arylation of spirocyclic σ1 ligands for analysis of complementary σ1 receptor surface. Eur. J. Org. Chem. 5972–5979 (2012).
Beydoun, K., Zaarour, M., Williams, J. A. G., Doucet, H. & Guerchais, V. Palladium-catalysed direct arylation of a tris-cyclometallated Ir(III) complex bearing 2,2′-thienylpyridine ligands: a powerful tool for the tuning of luminescence properties. Chem. Commun. 48, 1260–1262 (2012).
Nakazono, S. et al. Regioselective Ru-catalyzed direct 2,5,8,11-alkylation of perylene bisimides. Chem. Eur. J. 15, 7530–7533 (2009).
Nakazono, S., Easwaramoorthi, S., Kim, D., Shinokubo, H. & Osuka, A. Synthesis of arylated perylene bisimides through C–H bond cleavage under ruthenium catalysis. Org. Lett. 11, 5426–5429 (2009).
Teraoka, T., Hiroto, S. & Shinokubo, H. Iridium-catalyzed direct tetraborylation of perylene bisimides. Org. Lett. 13, 2532–2535 (2011).
Battagliarin, G., Davies, M., Mackowiak, S., Li, C. & Müllen, K. Ortho-functionalized perylenediimides for highly fluorescent water-soluble dyes. ChemPhysChem 13, 923–926 (2012).
Cohen, S. M. Postsynthetic methods for the functionalization of metal-organic frameworks. Chem. Rev. 112, 970–1000 (2012).
Dröge, T., Notzon, A., Fröhlich, R. & Glorius, F. Palladium-catalyzed C–H bond functionalization of a metal-organic framework (MOF): Mild, selective, and efficient. Chem. Eur. J. 17, 11974–11977 (2011).
Chen, H., Schlecht, S., Semple, T. C. & Hartwig, J. F. Thermal, catalytic, regiospecific functionalization of alkanes. Science 287, 1995–1997 (2000).
Hartwig, J. F. Borylation and silylation of C–H bonds: A platform for diverse C–H bond functionalizations. Acc. Chem. Res. 45, 864–873 (2012).
Kondo, Y. et al. Rhodium-catalyzed, regiospecific functionalization of polyolefins in the melt. J. Am. Chem. Soc. 124, 1164–1165 (2002).
Bae, C. et al. Catalytic hydroxylation of polypropylenes. J. Am. Chem. Soc. 127, 767–776 (2005).
Bae, C. et al. Regiospecific side-chain functionalization of linear low-density polyethylene with polar groups. Angew. Chem. Int. Ed. 44, 6410–6413 (2005).
Boaen, N. K. & Hillmyer, M. A. Post-polymerization functionalization of polyolefins. Chem. Soc. Rev. 34, 267–275 (2005).
Shin, J. et al. Controlled functionalization of crystalline polystyrenes via activation of aromatic C–H bonds. Macromolecules 40, 8600–8608 (2007).
Jo, T. S., Kim, S. H., Shin, J. & Bae, C. Highly efficient incorporation of functional groups into aromatic main-chain polymer using iridium-catalyzed C–H Activation and Suzuki–Miyaura reaction. J. Am. Chem. Soc. 131, 1656–1657 (2009).
Shimizu, M. & Hiyama, T. Silicon-bridged biaryls: Molecular design, new synthesis, and luminescence control. Synlett 23, 973–989 (2012).
Matsuda, T., Kadowaki, S., Goya, T. & Murakami, M. Synthesis of silafluorenes by iridium-catalyzed [2+2+2] cycloaddition of silicon-bridged diynes with alkynes. Org. Lett. 9, 133–136 (2007).
Shimizu, M., Mochida, K. & Hiyama, T. Modular approach to silicon-bridged biaryls: palladium-catalyzed intramolecular coupling of 2-(arylsilyl)aryl triflates. Angew. Chem. Int. Ed. 47, 9760–9764 (2008).
Mochida, K., Shimizu, M. & Hiyama, T. Palladium-catalyzed intramolecular coupling of 2-[(2-pyrrolyl)silyl]aryl triflates through 1,2-silicon migration. J. Am. Chem. Soc. 131, 8350–8351 (2009).
Shintani, R., Otomo, H., Ota, K. & Hayashi, T. Palladium-catalyzed asymmetric synthesis of silicon-stereogenic dibenzosiloles via enantioselective C–H bond functionalization. J. Am. Chem. Soc. 134, 7305–7308 (2012).
Schipper, D. J. & Fagnou, K. Direct arylation as a synthetic tool for the synthesis of thiophene-based organic electronic materials. Chem. Mater. 23, 1594–1600 (2011).
Mushrush, M., Facchetti, A., Lefenfeld, M., Katz, H. E. & Marks, T. J. Easily processable phenylene-thiophene-based organic field-effect transistors and solution-fabricated nonvolatile transistor memory elements. J. Am. Chem. Soc. 125, 9414–9423 (2003).
He, C.-Y., Fan, S. & Zhang X. Pd-catalyzed oxidative cross-coupling of perfluoroarenes with aromatic heterocycles. J. Am. Chem. Soc. 132, 12850–12852 (2010).
Wang, Q., Takita, R., Kikuzaki, Y. & Ozawa, F. Palladium-catalyzed dehydrohalogenative polycondensation of 2-bromo-3-hexylthiopene: An efficient approach to head-to-tail poly(3-hexylthiophene). J. Am. Chem. Soc. 132, 11420–11421 (2010).
Yokoyama, A., Miyakoshi, R. & Yokozawa, T. Chain-growth polymerization for poly(3-hexylthiophene) with a defined molecular weight and a low polydispersity. Macromolecules 37, 1169–1171 (2004).
Miyasaka, M., Fukushima, A., Satoh, T., Hirano, K. & Miura, M. Fluorescent diarylindoles by palladium-catalyzed direct and decarboxylative arylations of carboxyindoles. Chem. Eur. J. 15, 3674–3677 (2009).
Feng, X., Pisula, W. & Müllen, K. Large polycyclic aromatic hydrocarbons: Synthesis and discotic organization. Pure Appl. Chem. 81, 2203–2224 (2009).
Shimizu, M., Nagao, I., Tomioka, Y. & Hiyama, T. Palladium-catalyzed annulation of vic-bis(pinacolatoboryl)alkenes and –phenanthrenes with 2,2′-dibromobiaryls: facile synthesis of functionalized phenanthrenes and dibenzo[g, p]chrysenes. Angew. Chem. Int. Ed. 47, 8096–8099 (2008).
Mochida, K., Kawasumi, K., Segawa, Y. & Itami, K. Direct arylation of polycyclic aromatic hydrocarbons through palladium catalysis. J. Am. Chem. Soc. 133, 10716–10719 (2011).
Kawasumi, K., Mochida, K., Kajino, T., Segawa, Y. & Itami, K. Pd(OAc)2/o-Chloroanil/M(OTf)n: A catalyst for the direct C–H arylation of polycyclic aromatic hydrocarbons with boryl-, silyl-, and unfunctionalized arenes. Org. Lett. 14, 418–421 (2012).
Zhang, Q., Kawasumi, K., Segawa, Y., Itami, K. & Scott, L. T. Palladium-catalyzed C–H activation taken to the limit. Flattening an aromatic bowl by total arylation. J. Am. Chem. Soc. 134, 15664–15667 (2012).
Shi, Z., Ding, S., Cui, Y. & Jiao, N. A Palladium-catalyzed oxidative cycloaromatization of biaryls with alkynes using molecular oxygen as the oxidant. Angew. Chem. Int. Ed. 48, 7895–7898 (2009).
Umeda, N., Tsurugi, H., Satoh, T & Miura, M. Fluorescent naphthyl- and anthrylazoles from the catalytic coupling of phenylazoles with internal alkynes through the cleavage of multiple C–H bonds. Angew. Chem. Int. Ed. 47, 4019–4022 (2008).
Wu, Y.-T., Huang, K.-H., Shin, C.-C. & Wu, T.-C. Palladium-catalyzed formation of highly substituted naphthalenes from arene and alkyne hydrocarbons. Chem. Eur. J. 14, 6697–6703 (2008).
Kaneko, H., Nagae, H., Tsurugi, H. & Mashima, K. End-functionalized polymerization of 2-vinylpyridine through initial C–H bond activation of N-heteroaromatics and internal alkynes by yttrium ene–diamido complexes. J. Am. Chem. Soc. 133, 19626–19629 (2011).
Acknowledgements
We thank Karl Collins for helpful discussions. This work was supported by the European Research Council under the European Community's Seventh Framework Program (FP7 2007-2013)/ERC Grant agreement no 25936.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wencel-Delord, J., Glorius, F. C–H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nature Chem 5, 369–375 (2013). https://doi.org/10.1038/nchem.1607
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1607
This article is cited by
-
Enabling late-stage drug diversification by high-throughput experimentation with geometric deep learning
Nature Chemistry (2024)
-
Disulfide Bridged Two-Dimensional Erythrosine-B Polymer as a Tool for Photo-Catalytic C–H Activation
Catalysis Surveys from Asia (2024)
-
Organo–metal cooperative catalysis for C(sp3)–H alkylation polymerization
Nature Synthesis (2023)
-
Late-stage synthesis of heterobifunctional molecules for PROTAC applications via ruthenium-catalysed C‒H amidation
Nature Communications (2023)
-
Radical arenes
Nature Chemistry (2023)