Abstract
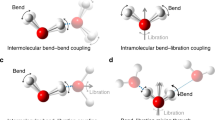
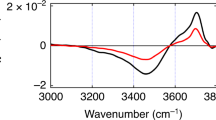
The ability of liquid water to dissipate energy efficiently through ultrafast vibrational relaxation plays a key role in the stabilization of reactive intermediates and the outcome of aqueous chemical reactions. The vibrational couplings that govern energy relaxation in H2O remain difficult to characterize because of the limitations of current methods to visualize inter- and intramolecular motions simultaneously. Using a new sub-70 fs broadband mid-infrared source, we performed two-dimensional infrared, transient absorption and polarization anisotropy spectroscopy of H2O by exciting the OH stretching transition and characterizing the response from 1,350 cm−1 to 4,000 cm−1. These spectra reveal vibrational transitions at all frequencies simultaneous to the excitation, including pronounced cross-peaks to the bend vibration and a continuum of induced absorptions to combination bands that are not present in linear spectra. These observations provide evidence for strong mixing of inter- and intramolecular vibrations in liquid H2O, and illustrate the shortcomings of traditional relaxation models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pakoulev, A., Wang, Z. & Dlott, D. D. Vibrational relaxation and spectral evolution following ultrafast OH stretch excitation of water. Chem. Phys. Lett. 371, 594–600 (2003).
Lindner, J., Cringus, D., Pshenichnikov, M. S. & Vöhringer, P. Anharmonic bend–stretch coupling in neat liquid water. Chem. Phys. 341, 326–335 (2007).
Lindner, J. et al. Vibrational relaxation of pure liquid water. Chem. Phys. Lett. 421, 329–333 (2006).
Wang, Z., Pakoulev, A., Pang, Y. & Dlott, D. D. Vibrational substructure in the OH stretching transition of water and HOD. J. Phys. Chem. A 108, 9054–9063 (2004).
Larsen, O. F. A. & Woutersen, S. Vibrational relaxation of the H2O bending mode in liquid water. J. Chem. Phys. 121, 12143–12145 (2004).
Cowan, M. L. et al. Ultrafast memory loss and energy redistribution in the hydrogen bond network of liquid H2O. Nature 434, 199–202 (2005).
Huse, N., Ashihara, S., Nibbering, E. T. J. & Elsaesser, T. Ultrafast vibrational relaxation of O–H bending and librational excitations in liquid H2O. Chem. Phys. Lett. 404, 389–393 (2005).
Deàk, J. C., Rhea, S. T., Iwaki, L. K. & Dlott, D. D. Vibrational energy relaxation and spectral diffusion in water and deuterated water. J. Phys. Chem. A 104, 4866–4875 (2000).
Kraemer, D. et al. Temperature dependence of the two-dimensional infrared spectrum of liquid H2O. Proc. Natl Acad. Sci. USA 105, 437–442 (2008).
Lock, A. J. & Bakker, H. J. Temperature dependence of vibrational relaxation in liquid H2O. J. Chem. Phys. 117, 1708–1713 (2002).
Ashihara, S., Huse, N., Espagne, A., Nibbering, E. T. J. & Elsaesser, T. Ultrafast structural dynamics of water induced by dissipation of vibrational energy. J. Phys. Chem. A 111, 743–746 (2007).
Rey, R. & Hynes, J. T. Tracking energy transfer from excited to accepting modes: application to water bend vibrational relaxation. Phys. Chem. Chem. Phys. 14, 6332–6342 (2012).
Jansen, T. L. C., Auer, B. M., Yang, M. & Skinner, J. L. Two-dimensional infrared spectroscopy and ultrafast anisotropy decay of water. J. Chem. Phys. 132, 224503 (2010).
Yagasaki, T. & Saito, S. A novel method for analyzing energy relaxation in condensed phases using nonequilibrium molecular dynamics simulations: application to the energy relaxation of intermolecular motions in liquid water. J. Chem. Phys. 134, 184503 (2011).
Auer, B. M. & Skinner, J. L. IR and Raman spectra of liquid water: theory and interpretation. J. Chem. Phys. 128, 224511 (2008).
Paarmann, A., Hayashi, T., Mukamel, S. & Miller, R. J. D. Nonlinear response of vibrational excitons: simulating the two-dimensional infrared spectrum of liquid water. J. Chem. Phys. 130, 204110 (2009).
Falvo, C., Palmieri, B. & Mukamel, S. Coherent infrared multidimensional spectra of the OH stretching band in liquid water simulated by direct nonlinear exciton propagation. J. Chem. Phys. 130, 184501 (2009).
Hale, G. M. & Querry, M. R. Optical constants of water in the 200 nm to 200 µm wavelength region. Appl. Opt. 12, 555–63 (1973).
Woutersen, S. & Bakker, H. J. Resonant intermolecular transfer of vibrational energy in liquid water. Nature 402, 507–509 (1999).
De Marco, L., Ramasesha, K. & Tokmakoff, A. Experimental evidence for Fermi resonances in isotopically dilute water from ultrafast IR spectroscopy. J. Phys. Chem. B doi:10.1021/jp4034613 (2013).
Libnau, F. O., Kvalheim, O. M., Christy, A. A. & Toft, J. Spectra of water in the near- and mid-infrared region. Vib. Spec. 7, 243–254 (1994).
Yang, M., Li, F. & Skinner, J. L. Vibrational energy transfer and anisotropy decay in liquid water: is the Förster model valid? J. Chem. Phys. 135, 164505 (2011).
Perakis, F., Widmer, S. & Hamm, P. Two-dimensional infrared spectroscopy of isotope-diluted ice Ih. J. Chem. Phys. 134, 204505 (2011).
Hamm, P. & Stock, G. Vibrational conical intersections as a mechanism of ultrafast vibrational relaxation. Phys. Rev. Lett. 109, 1–5 (2012).
Petersen, P. B. & Tokmakoff, A. Source for ultrafast continuum infrared and terahertz radiation. Opt. Lett. 35, 1962–1964 (2010).
Fecko, C. J., Loparo, J. J. & Tokmakoff, A. Generation of 45 femtosecond pulses at 3 μm with a KNbO optical parametric amplifier. Opt. Commun. 241, 521–528 (2004).
Deflores, L. P., Nicodemus, R. A. & Tokmakoff, A. Two-dimensional Fourier transform spectroscopy in the pump–probe geometry. Opt. Lett. 32, 2966–2968 (2007).
Acknowledgements
This work was supported by the US Department of Energy (DE-FG02-99ER14988). L.D.M thanks the Natural Sciences and Engineering Research Council of Canada for a scholarship.
Author information
Authors and Affiliations
Contributions
A.T., K.R. and L.D.M. conceived and designed experiments. K.R., L.D.M. and A.M. constructed the spectrometer and performed the experiments. A.T., K.R., L.D.M. and A.M. analysed results. K.R. and A.T. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ramasesha, K., De Marco, L., Mandal, A. et al. Water vibrations have strongly mixed intra- and intermolecular character. Nature Chem 5, 935–940 (2013). https://doi.org/10.1038/nchem.1757
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1757
This article is cited by
-
Unified picture of vibrational relaxation of OH stretch at the air/water interface
Nature Communications (2024)
-
Two-dimensional infrared-Raman spectroscopy as a probe of water’s tetrahedrality
Nature Communications (2023)
-
A six-octave optical frequency comb from a scalable few-cycle erbium fibre laser
Nature Photonics (2021)
-
Direct observation of ultrafast hydrogen bond strengthening in liquid water
Nature (2021)
-
Infrared spectroscopy data- and physics-driven machine learning for characterizing surface microstructure of complex materials
Nature Communications (2020)