Abstract
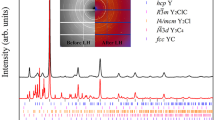
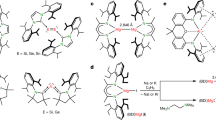
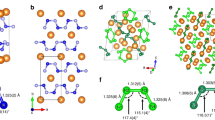
The periodicity of the elements and the non-reactivity of the inner-shell electrons are two related principles of chemistry, rooted in the atomic shell structure. Within compounds, Group I elements, for example, invariably assume the +1 oxidation state, and their chemical properties differ completely from those of the p-block elements. These general rules govern our understanding of chemical structures and reactions. Here, first-principles calculations show that, under pressure, caesium atoms can share their 5p electrons to become formally oxidized beyond the +1 state. In the presence of fluorine and under pressure, the formation of CsFn (n > 1) compounds containing neutral or ionic molecules is predicted. Their geometry and bonding resemble that of isoelectronic XeFn molecules, showing a caesium atom that behaves chemically like a p-block element under these conditions. The calculated stability of the CsFn compounds shows that the inner-shell electrons can become the main components of chemical bonds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Landau, L. D. & Lifshitz, E. M. in Quantum Mechanics (Non-Relativistic Theory) Vol. 3 (Butterworth-Heinemann, 1958).
Pauling, L. The Nature of the Chemical Bond (Cornell Univ. Press, 1960).
Murrel, J. N., Kettle, S. F. A. & Tedder, J. M. in The Chemical Bond (Wiley, 1985).
Bartlett, N. Xenon hexafluoroplatinate (V) Xe+PtF6 . Proc. Chem. Soc. Lond. (1962).
Agron, P. A. et al. Xenon difluoride and nature of xenon–fluorine bond. Science 139, 842–844 (1963).
Siegel, S. & Gebert, E. Crystallographic studies of XeF2 and XeF4 . J. Am. Chem. Soc. 85, 240 (1963).
Rundle, R. E. Probable structure of XeF4 and XeF2 . J. Am. Chem. Soc. 85, 112 (1963).
Malm, J. G. et al. Xenon hexafluoride. J. Am. Chem. Soc. 85, 110–111 (1963).
Liao, M. S. & Zhang, Q. E. Chemical bonding in XeF2, XeF4, KrF2, KrF4, RnF2, XeCl2, and XeBr2: from the gas phase to the solid state. J. Phys. Chem. A 102, 10647–10654 (1998).
Krouse, I. H. et al. Bonding and electronic structure of XeF3 . J. Am. Chem. Soc. 129, 846–852 (2007).
Khriachtchev, L., Pettersson, M., Runeberg, N., Lundell, J. & Rasanen, M. A stable argon compound. Nature 406, 874–876 (2000).
Grochala, W. Atypical compounds of gases, which have been called ‘noble’. Chem. Soc. Rev. 36, 1632–1655 (2007).
Dmochowski, I. Xenon out of its shell. Nature Chem. 1, 250 (2009).
Brock, D. S. & Schrobilgen, G. Synthesis of the missing oxide of xenon, XeO2, and its implications for Earth's missing xenon. J. Am. Chem. Soc. 133, 6265–6269 (2011).
Kim, M., Debessai, M. & Yoo, C. S. Two- and three-dimensional extended solids and metallization of compressed XeF2 . Nature Chem. 2, 784–788 (2010).
Bode, H. Uber einige neue fluoraktive Stoffe. Naturwissenschaften 37, 477 (1950).
Moock, K. & Seppelt, K. Indications of cesium in a higher oxidation-state. Angew. Chem. Int. Ed. Engl. 28, 1676–1678 (1989).
Asprey, L. B., Margrave, J. L. & Silverthorne, M. E. Tetrafluorohalates of cesium, rubidium and potassium. J. Am. Chem. Soc. 83, 2955–2956 (1961).
Jehoulet, C. & Bard, A. J. On the electrochemical oxidation of Cs+ and other alkali-metal ions in liquid sulfur dioxide and acetonitrile. Angew. Chem. Int. Ed. Engl. 30, 836–838 (1991).
Schwarz, U., Takemura, K., Hanfland, M. & Syassen, K. Crystal structure of cesium-V. Phys. Rev. Lett. 81, 2711–2714 (1998).
Takemura, K. et al. Phase stability of highly compressed cesium. Phys. Rev. B 61, 14399–14404 (2000).
Takemura, K., Minomura, S. & Shimomura, O. X-ray diffraction study of electronic-transitions in cesium under high pressure. Phys. Rev. Lett. 49, 1772–1775 (1982).
Shamp, A., Hooper, J. & Zurek, E. Compressed cesium polyhydrides: Cs+ sublattices and H3− three-connected nets. Inorg. Chem. 51, 9333–9342 (2012).
Hooper, J. & Zurek, E. Rubidium polyhydrides under pressure: emergence of the linear H3− species. Chem. Eur. J. 18, 5013–5021 (2012).
Johnson, K. A. & Ashcroft, N. W. Structure and bandgap closure in dense hydrogen. Nature 403, 632–635 (2000).
Belonoshko, A. B., Ahuja, R. & Johansson, B. Stability of the body-centred-cubic phase of iron in the Earth's inner core. Nature 424, 1032–1034 (2003).
Oganov, A. R. et al. Ionic high-pressure form of elemental boron. Nature 457, 863–867 (2009).
Ma, Y. et al. Transparent dense sodium. Nature 458, 182–U183 (2009).
Wang, Y. C. et al. High pressure partially ionic phase of water ice. Nature Commun. 2, 563 (2011).
Zhu, L. et al. Spiral chain O4 form of dense oxygen. Proc. Natl Acad. Sci. USA 109, 751–753 (2012).
Wang, Y. C., Lv, J., Zhu, L. & Ma, Y. M. CALYPSO: a method for crystal structure prediction. Comput. Phys. Commun. 183, 2063–2070 (2012).
Wang, Y. C., Lv, J. A., Zhu, L. & Ma, Y. M. Crystal structure prediction via particle-swarm optimization. Phys. Rev. B 82, 094116 (2010).
Peng, F., Miao, M. S., Wang, H., Li, Q. & Ma, Y. M. Predicted lithium–boron compounds under high pressure. J. Am. Chem. Soc. 134, 18599–18605 (2012).
Feng, J., Hennig, R. G., Ashcroft, N. W. & Hoffmann, R. Emergent reduction of electronic state dimensionality in dense ordered Li–Be alloys. Nature 451, 445–448 (2008).
Ault, B. S. & Andrews, L. Matrix reactions of alkali-metal fluoride molecules with fluorine—infrared and Raman-spectra of trifluoride ion in M+F3−-species. J. Am. Chem. Soc. 98, 1591–1593 (1976).
Riedel, S., Koechner, T., Wang, X. F. & Andrews, L. Polyfluoride anions, a matrix-isolation and quantum-chemical investigation. Inorg. Chem. 49, 7156–7164 (2010).
Christe, K. O. et al. The pentafluoroxenate(IV) anion, XeF5−—the first example of a pentagonal planar AX5 species. J. Am. Chem. Soc. 113, 3351–3361 (1991).
Dronskowski, R. & Blochl, P. E. Crystal orbital Hamiltonian populations (COHP)—energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 97, 8617–8624 (1993).
Braida, B. & Hiberty, P. C. The essential role of charge-shift bonding in hypervalent prototype XeF2 . Nature Chem. 5, 417–422 (2013).
Silvi, B. & Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 371, 683–686 (1994).
Bader, R. Atoms in Molecules: A Quantum Theory (Oxford Univ. Press, 1990).
Zurek, E., Hoffmann, R., Ashcroft, N. W., Oganov, A. R. & Lyakhov, A. O. A little bit of lithium does a lot for hydrogen. Proc. Natl Acad. Sci. USA 106, 17640–17643 (2009).
Baettig, P. & Zurek, E. Pressure-stabilized sodium polyhydrides: NaHn (n > 1). Phys. Rev. Lett. 106, 237002 (2011).
Zhang, W. et al. Unexpected stable stoichiometries of sodium chlorides. Preprint at http://arxiv.org/1211.3644 (2012).
Zhu, Q., Oganov, A. R. & Lyakhov, A. O. Novel stable compounds in the Mg–O system under high pressure. Phys. Chem. Chem. Phys. 15, 7696–7700 (2013).
Tatsumi, K. & Hoffmann, R. Bent cis d0 MOO22+ vs linear trans d0f0 UO22+—a significant role for non-valence 6p orbitals in uranyl. Inorg. Chem. 19, 2656–2658 (1980).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Acknowledgements
The author thanks R. Hoffmann at Cornell University and R. Seshadri at University of California Santa Barbara for inspiring discussions and constructive suggestions. This work was supported by the Materials Research Science and Engineering Center programme (National Science Foundation (NSF)-Division of Materials Research (DMR)1121053) and the Conversion of Energy Through Molecular Platforms-The Integrative Graduate Education and Research Traineeship programme (NSF-Division of Graduate Education (DGE)0801627). Calculations were performed on resources at the Center for Scientific Computing, supported by the California NanoSystems Institute, Materials Research Lab. and NSF (Division of Computer and Network Systems (CNS)-0960316), on NSF-funded Extreme Science and Engineering Discovery Environment resources (Teragrid (TG)-DMR130005) and on Beijing Computational Science Research Center computing resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1341 kb)
Rights and permissions
About this article
Cite this article
Miao, Ms. Caesium in high oxidation states and as a p-block element. Nature Chem 5, 846–852 (2013). https://doi.org/10.1038/nchem.1754
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1754
This article is cited by
-
Open questions on the high-pressure chemistry of the noble gases
Communications Chemistry (2022)
-
Ag9GaSe6: high-pressure-induced Ag migration causes thermoelectric performance irreproducibility and elimination of such instability
Nature Communications (2022)
-
Formation of twelve-fold iodine coordination at high pressure
Nature Communications (2022)
-
Materials under high pressure: a chemical perspective
Applied Physics A (2022)
-
Superconducting ScP4 with a novel phosphorus framework
Applied Physics A (2022)