Abstract
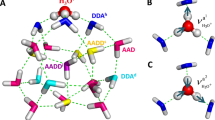
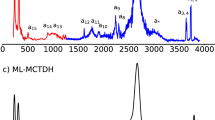
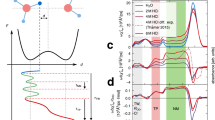
In liquid water the transfer of an excess proton between two water molecules occurs through the Zundel cation, H2O···H+···OH2. The proton-transfer mode is the asymmetric stretch of the central O···H+···O moiety, but there is no consensus on its identification in the infrared spectra of acidic aqueous solutions. Also, in experiments with protonated gas-phase water clusters, its position shifts with cluster size, which makes its relationship with solution spectra unclear. Here we introduce a ‘clusters-in-liquid’ approach for calculating the infrared spectrum from any set of charges, even single protons. We apply this procedure to multistate empirical valence-bond trajectories of protonated liquid water and to ab initio molecular dynamics of the protonated water dimer and hexamer in the gas phase. The calculated proton-transfer mode is manifested in both systems as a peak near 1,740 cm−1, in quantitative agreement with a band of similar frequency in the experimental infrared spectrum of protonated water clusters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wraight, C. A. Chance and design – proton transfer in water, channels and bioenergetic proteins. Biochim. Biophys. Acta 1757, 886–912 (2006).
Kreuer, K-D., Paddison, S. J., Spohr, E. & Schuster, M. Transport in proton conductors for fuel-cell applications: simulations, elementary reactions, and phenomenology. Chem. Rev. 104, 4637–4678 (2004).
Agmon, N. & Gutman, M. Proton solvation and proton mobility. Isr. J. Chem. 39 (Special Issue) (1999).
de Grotthuss, C. J. T. Sur la décomposition de l'eau et de corps qu'elle tient en dissolution à l'aide de l’électricité galvanique. Ann. Chim. LVIII, 54–74 (1806).
Cukierman, S. Et tu, Grotthuss! and other unfinished stories. Biochim. Biophys. Acta 1757, 876–885 (2006).
Hückel, E. Theory of the mobilities of the H and OH ions in aqueous solutions. Z. Electrochem. 34, 546–562 (1928).
Wicke, E., Eigen, M. & Ackermann, T. Über den Zustand des Protons (Hydroniumions) in wäßriger Lösung. Z. Phys. Chem. 1, 340–364 (1954).
Markovitch, O. & Agmon, N. Structure and energetics of the hydronium hydration shells. J. Phys. Chem. A 111, 2253–2256 (2007).
Zundel, G. Hydration structure and intermolecular interaction in polyelectrolytes. Ang. Chem. Int. Ed. Engl. 8, 499–509 (1969).
Zundel, G. in The Hydrogen Bond, Recent Developments in Theory and Experiments (eds Schuster, P., Zundel, G. & Sandorfy, C.) 687–766 (North Holland, 1976).
Agmon, N., Goldberg, S. Y. & Huppert, D. Salt effect on transient proton transfer to solvent and microscopic proton mobility. J. Mol. Liq. 64, 161–195 (1995).
Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 244, 456–462 (1995).
Markovitch, O. et al. Special pair dance and partner selection: elementary steps in proton transport in liquid water. J. Phys. Chem. B 112, 9456–9466 (2008).
Marx, D., Tuckerman, M. E., Hutter, J. & Parrinello, M. The nature of the hydrated excess proton in water. Nature 397, 601–604 (1999).
Lapid, H., Agmon, N., Petersen, M. K. & Voth, G. A. A bond-order analysis of the mechanism for hydrated proton mobility in liquid water. J. Chem. Phys. 122, 014506 (2005).
Tielrooij, K. J., Timmer, R. L. A., Bakker, H. J. & Bonn, M. Structure dynamics of the proton in liquid water probed with terahertz time-domain spectroscopy. Phys. Rev. Lett. 102, 198303 (2009).
Ackermann, T. Das Absorptionsspektrum wäßinger Säure- und Alkali-hydroxydlösungen im Wellenlängenbereich von 2,5 bis 9µ. Z. Phys. Chem. 27, 253–276 (1961).
Falk, M. & Giguère, P. A. Infrared spectrum of the H3O+ ion in aqueous solutions. Can. J. Chem. 35, 1195–1204 (1957).
Rhine, P., Williams, D., Hale, G. M. & Querry, M. R. Infrared optical constants of aqueous solutions of electrolytes. Acids and bases. J. Phys. Chem. 78, 1405–1410 (1974).
Downing, H. D. & Williams, D. Infrared spectra of strong acids and bases. J. Phys. Chem. 80, 1640–1641 (1976).
Librovich, N. B., Sakun, V. P. & Sokolov, N. D. H+ and OH− ions in aqueous solutions. Vibrational spectra of hydrates. Chem. Phys. 39, 351–366 (1979).
Śmiechowski, M. & Stangret, J. ATR FT-IR H2O spectra of acidic aqueous solutions. Insights about proton hydration. J. Mol. Struct. 878, 104–115 (2008).
Stoyanov, E. S., Stoyanova, I. V. & Reed, C. A. The structure of the hydrogen ion (Haq+) in water. J. Am. Chem. Soc. 132, 1484–1485 (2010).
Kim, J., Schmitt, U. W., Gruetzmacher, J. A., Voth, G. A. & Scherer, N. E. The vibrational spectrum of the hydrated proton: Comparison of experiment, simulation, and normal mode analysis. J. Chem. Phys. 116, 737–746 (2002).
Vuilleumier, R. & Borgis, D. Transport and spectroscopy of the hydrated proton: a molecular dynamics study. J. Chem. Phys. 111, 4251–4266 (1999).
Iftimie, R. & Tuckerman, M. E. Decomposing total IR spectra of aqueous systems into solute and solvent contributions: a computational approach using maximally localized Wannier orbitals. J. Chem. Phys. 122, 214508 (2005).
Iftimie, R. & Tuckerman, M. E. The molecular origin of the continuous infrared absorption in aqueous solutions of acids: a computational approach. Ang. Chem. Intl Ed. 45, 1144–1147 (2006).
Xu, J., Zhang, Y. & Voth, G. A. Infrared spectrum of the hydrated proton in water. J. Phys. Chem. Lett. 2, 81–86 (2011).
Asmis, K. R. et al. Gas-phase infrared spectrum of the protonated water dimer. Science 299, 1375–1377 (2003).
Headrick, J. M. et al. Spectral signatures of hydrated proton vibrations in water clusters. Science 308, 1765–1769 (2005).
Olesen, S. G., Guasco, T. L., Roscioli, J. R. & Johnson, M. A. Tuning the intermolecular proton bond in the H5O2+ ‘Zundel ion’ scaffold. Chem. Phys. Lett. 509, 89–95 (2011).
Vendrell, O., Gatti, F. & Meyer, H-D. Full dimensional (15-dimensional) quantum-dynamical simulation of the protonated water dimer. II. Infrared spectrum and vibrational dynamics. J. Chem. Phys. 127, 184303 (2007).
Niedner-Schatteburg, G. Infrared spectroscopy and ab initio theory of isolated H5O2+: from buckets of water to the Schrödinger equation and back. Ang. Chem. Intl Ed. 47, 1008–1011 (2008).
Agmon, N. Structure of concentrated HCl solutions. J. Phys. Chem. A 102, 192–199 (1998).
Agostini, F., Vuilleumier, R. & Ciccotti, G. Infrared spectroscopy of small protonated water clusters at room temperature: an effective modes analysis. J. Chem. Phys. 134, 084302 (2011).
Day, T. J. F., Soudackov, A. V., Čuma, M., Schmitt, U. W. & Voth, G. A. A second generation multistate empirical valence bond model for proton transport in aqueous systems. J. Chem. Phys. 117, 5839–5849 (2002).
Wu, Y., Chen, H., Wang, F., Paesani, F. & Voth, G. A. An improved multistate empirical valence bond model for aqueous proton solvation and transport. J. Phys. Chem. B 112, 467–482 (2008); erratum: 112, 7146 (2008).
Wu, Y., Tepper, H. L. & Voth, G. A. Flexible simple point-charge water model with improved liquid-state properties. J. Chem. Phys. 124, 024503 (2006).
Haese, N. N. & Oka, T. Observation of the ν2 (1− ← 0+) inversion mode band in H3O+ by high resolution infrared spectroscopy. J. Chem. Phys. 80, 572–573 (1984).
Nakamoto, K., Margoshes, M. & Rundle, R. E. Stretching frequencies as a function of distances in hydrogen bonds. J. Am. Chem. Soc. 77, 6480–6486 (1955).
Swanson, J. M. J. & Simons, J. Role of charge transfer in the structure and dynamics of the hydrated proton. J. Phys. Chem. B 113, 5149–5161 (2009).
Yu, H. & Cui, Q. The vibrational spectra of protonated water clusters: a benchmark for self-consistent-charge density-functional tight binding. J. Chem. Phys. 127, 234504 (2007).
Smith, W. & Forester, T. R. DL_POLY, version 2.13. CCLRC Daresbury Laboratory (Daresbury, 1999).
CP2K version 2.3 (CP2K Development Group, http://www.cp2k.org/).
Goedecker, S., Teter, M. & Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 54, 1703–1710 (1996).
Acknowledgements
We are indebted to G. A. Voth for a copy of the MS-EVB3 program and to M. A. Johnson and M. Śmiechowski for experimental data. This research was supported by the US–Israel Binational Science Foundation, grant numbers 2006067 and 2010250. The Fritz Haber Center is supported by the Minerva Gesellschaft für die Forschung, Munich.
Author information
Authors and Affiliations
Contributions
W.K. performed the calculations, prepared the figures and wrote a first draft of the manuscript. N.A. designed the research, interpreted the data and wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1875 kb)
Rights and permissions
About this article
Cite this article
Kulig, W., Agmon, N. A ‘clusters-in-liquid’ method for calculating infrared spectra identifies the proton-transfer mode in acidic aqueous solutions. Nature Chem 5, 29–35 (2013). https://doi.org/10.1038/nchem.1503
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1503
This article is cited by
-
The coupling of the hydrated proton to its first solvation shell
Nature Communications (2022)
-
Spectral signatures of excess-proton waiting and transfer-path dynamics in aqueous hydrochloric acid solutions
Nature Communications (2022)
-
Towards complete assignment of the infrared spectrum of the protonated water cluster H+(H2O)21
Nature Communications (2021)
-
Identifying Eigen-like hydrated protons at negatively charged interfaces
Nature Communications (2020)
-
Orientation of non-spherical protonated water clusters revealed by infrared absorption dichroism
Nature Communications (2018)