Abstract
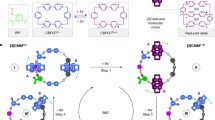
Imidazole, a subunit of histidine, plays a crucial role in proton-relay processes that are important for various biological activities, such as metal efflux, viral replication and photosynthesis. We show here how an imidazolyl ring incorporated into a rotary switch based on a hydrazone enables a switching cascade that involves proton relay between two different switches. The switching process starts with a single input, zinc(II), that initiates an E/Z isomerization in the hydrazone system through a coordination-coupled proton transfer. The resulting imidazolium ring is unusually acidic and, through proton relay, activates the E/Z isomerization of a non-coordinating pyridine-containing hydrazone switch. We hypothesize that the reduction in the acid dissociation constant of the imidazolium ring results from a combination of electrostatic and conformational effects, the study of which might help elucidate the proton-coupled electron-transfer mechanism in photosynthetic bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Williams, R. J. P. Proton circuits in biological energy interconversions. Annu. Rev. Biophys. Biophys. Chem. 17, 71–97 (1988).
Pinto, L. H. et al. A functionally defined model for the M2 proton channel of influenza A virus suggests a mechanism for its ion selectivity. Proc. Natl Acad. Sci. USA 94, 6183–6188 (1997).
Takeda, M., Pekosz, A., Shuck, K., Pinto, L. H. & Lamb, R. A. Influenza A virus M2 ion channel activity is essential for efficient replication in tissue culture. J. Virol. 76, 1391–1399 (2002).
Hu, F., Luo, W. & Hong, M. Mechanisms of proton conduction and gating in influenza M2 proton channels from solid-state NMR. Science 330, 505–508 (2010).
Feher, G., Allen, J. P., Okamura, M. Y. & Rees, D. C. Structure and function of bacterial photosynthetic reaction centres. Nature 339, 111–116 (1989).
Paddock, M., Graige, M., Feher, G. & Okamura, M. Identification of the proton pathway in bacterial reaction centers: inhibition of proton transfer by binding of Zn2+ or Cd2+. Proc. Natl Acad. Sci. USA 96, 6183–6188 (1999).
Utschig, L., Poluektov, O., Tiede, D. & Thurnauer, M. EPR investigation of Cu2+-substituted photosynthetic bacterial reaction centers: evidence for histidine ligation at the surface metal site. Biochemistry 39, 2961–2969 (2000).
Gerencsér, L. & Maróti, P. Retardation of proton transfer caused by binding of the transition metal ion to the bacterial reaction center is due to pKa shifts of key protonatable residues. Biochemistry 40, 1850–1860 (2001).
Wei, Y. & Fu, D. Selective metal binding to a membrane-embedded aspartate in the Escherichia coli metal transporter YiiP. J. Biol. Chem. 280, 33716–33724 (2005).
Wei, Y. & Fu, D. Binding and transport of metal ions at the dimer interface of the Escherichia coli metal transporter YiiP. J. Biol. Chem. 281, 23492–23502 (2006).
Ohana, E. et al. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 284, 17677–17686 (2009).
Dempsey, J. L., Winkler, J. R. & Gray, H. B. Proton-coupled electron flow in protein redox machines. Chem. Rev. 110, 7024–7039 (2010).
Kaila, V. R. I., Verkhovsky, M. I. & Wikström, M. Proton-coupled electron transfer in cytochrome oxidase. Chem. Rev. 110, 7062–7081 (2010).
Nagle, J. F. & Morowitz, H. Molecular mechanisms for proton transport in membranes. J. Proc. Natl Acad. Sci. USA 75, 298–302 (1978).
Le Duc, Y. et al. Imidazole-quartet water and proton dipolar channels. Angew. Chem. Int. Ed. 50, 11366–11372 (2011).
Chen, Y. et al. Enhancement of anhydrous proton transport by supramolecular nanochannels in comb polymers. Nature Chem. 2, 503–508 (2010).
Feringa, B. L. Molecular Switches (Wiley-VCH, 2001).
Kay, E. R., Leigh, D. A. & Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 46, 72–191 (2007).
Balzani, V., Credi, A. & Venturi, M. Molecular Devices and Machines – Concepts and Perspectives for the Nanoworld (Wiley-VCH, 2008).
Stoddart, J. F. The chemistry of the mechanical bond. Chem. Soc. Rev. 38, 1802–1820 (2009).
Breslow, R. Biomimetic chemistry: biology as an inspiration. J. Biol. Chem. 284, 1337–1342 (2009).
Landge, S. M. & Aprahamian, I. pH activated configurational rotary switch: controlling the E/Z isomerization in hydrazones. J. Am. Chem. Soc. 131, 18269–18271 (2009).
Su, X. & Aprahamian, I. Switching around two axles: controlling the configuration and conformation of a hydrazone-based switch. Org. Lett. 13, 30–33 (2011).
Landge, S. M. et al. Isomerization mechanism in hydrazone-based rotary switches: lateral shift, rotation, or tautomerization? J. Am. Chem. Soc. 133, 9812–9823 (2011).
Su, X., Robbins, T. F. & Aprahamian, I. Switching through coordination-coupled proton transfer. Angew. Chem. Int. Ed. 50, 1841–1844 (2011).
Schliwa, M. Molecular Motors (VCH-Wiley, 2003).
Macco, A. A., Godefroi, E. F. & Drouen, J. J. M. 2-(2-imidazolyl)acetophenones. Preparation and some reactions. J. Org. Chem. 40, 252–255 (1975).
Pavlović, G., Racané, L., Čičak, H. & Tralić-Kulenović, V. The synthesis and structural study of two benzothiazolyl azo dyes: X-ray crystallographic and computational study of azo–hydrazone tautomerism. Dyes Pigments 83, 354–362 (2009).
Gernencer, L., Taly, A., Baciou, L., Maroti, P. & Sebban, P. Effect of binding of Cd2+ on bacterial reaction center mutants: proton-transfer uses interdependent pathways. Biochemistry 41, 9132–9138 (2002).
Utschig, L. M. & Thurnauer, M. C. Metal ion modulated electron transfer in photosynthetic bacteria. Acc. Chem. Res. 37, 439–447 (2004).
Paddock, M. et al. Mechanism of proton transfer inhibition by Cd2+ binding to bacterial reaction centers: determination of the pKa of functionally important histidine residues. Biochemistry 42, 9626–9632 (2002).
Ishikita, H. & Knapp, E-W. Induced conformational changes upon Cd2+ binding at photosynthetic reaction centers. Proc. Natl Acad. Sci. USA 102, 16215–16220 (2005).
Jaguar 7.7 (Schrödinger, New York, 2010).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Hay, P. J. & Wadt, W. R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 82, 270–283 (1985).
Huque, F. T. T. & Platts, J. A. The effects of intramolecular interactions on hydrogen bond acidity. Org. Biomol. Chem. 1, 1419–1423 (2003).
Ball, P. Welcome to the machine. Chem. World 7, 56–60 (2010).
Astumian, R. D. & Robertson, B. Imposed oscillations of kinetic barriers can cause an enzyme to drive a chemical reaction away from equilibrium. J. Am. Chem. Soc. 115, 11063–11068 (1993).
von Delius, M., Geertsema, E. M. & Leigh, D. A. A synthetic small molecule that can walk down a track. Nature Chem. 2, 96–101 (2010).
Wang, J. & Feringa, B. L. Dynamic control of chiral space in a catalytic asymmetric reaction using a molecular motor. Science 331, 1429–1432 (2011).
Coskun, A., Banaszak, M., Astumian, R. D., Stoddart, J. F. & Grzybowski, B. A. Great expectations: can artificial molecular machines deliver on their promise? Chem. Soc. Rev. 41, 19–30 (2012).
Whitesides, G. M. & Ismagilov, R. F. Complexity in chemistry. Science 284, 89–92 (1999).
Devanathan, R. Recent developments in proton exchange membranes for fuel cells. Energy. Environ. Sci. 1, 101–119 (2008).
Dubois, M. R. & Dubois, D. L. Development of molecular electrocatalysts for CO2 reduction and H2 production/oxidation. Acc. Chem. Res. 42, 1974–1982 (2009).
Acknowledgements
The authors dedicate this manuscript to Sir Fraser Stoddart on the occasion of his 70th birthday. This work was supported by Dartmouth College, the Burke Research Initiation Award and the American Chemical Society Petroleum Research Fund. The authors thank R. Staples for the X-ray analysis, S. Voskian for his help with the graphic for the table of contents and D.S. Glueck for his input.
Author information
Authors and Affiliations
Contributions
All experiments were conducted by D.R. and J.T.F. with input from I.A. The calculations were carried out and interpreted by R.P.H. The manuscript was co-written by I.A. and D.R. with input from R.P.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 3531 kb)
Supplementary information
Crystallographic data for compound QIH-Me (CIF 17 kb)
Supplementary information
Crystallographic data for compound QIH-Me(Z-H+) (CIF 20 kb)
Supplementary information
Crystallographic data for compound Zn(QIH-Me(Z-H+) (CIF 22 kb)
Supplementary information
Crystallographic data for compound Zn(QPH-Me(Z-H+)) (CIF 23 kb)
Rights and permissions
About this article
Cite this article
Ray, D., Foy, J., Hughes, R. et al. A switching cascade of hydrazone-based rotary switches through coordination-coupled proton relays. Nature Chem 4, 757–762 (2012). https://doi.org/10.1038/nchem.1408
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1408
This article is cited by
-
Unexpected organic hydrate luminogens in the solid state
Nature Communications (2021)
-
2-Nitro- and 4-fluorocinnamaldehyde based receptors as naked-eye chemosensors to potential molecular keypad lock
Scientific Reports (2021)
-
A curved host and second guest cooperatively inhibit the dynamic motion of corannulene
Nature Communications (2021)
-
Stereodivergent synthesis with a programmable molecular machine
Nature (2017)
-
Reversible proton-switchable fluorescence controlled by conjugation effect in an organically-functionalized polyoxometalate
Scientific Reports (2016)