Abstract
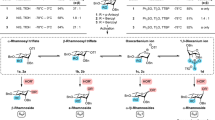
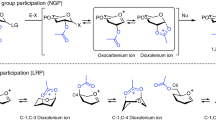
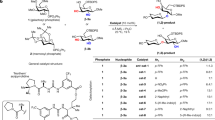
Although arguably the most important reaction in glycoscience, chemical glycosylations are among the least well understood of organic chemical reactions, resulting in an unnecessarily high degree of empiricism and a brake on rational development in this critical area. To address this problem, primary 13C kinetic isotope effects have now been determined for the formation of β- and α-manno- and glucopyranosides using a natural abundance NMR method. In contrast to the common current assumption, for three of the four cases studied the experimental and computed values are indicative of associative displacement of the intermediate covalent glycosyl trifluoromethanesulfonates. For the formation of the α-mannopyranosides, the experimentally determined KIE differs significantly from that computed for an associative displacement, which is strongly suggestive of a dissociative mechanism that approaches the intermediacy of a glycosyl oxocarbenium ion. The application of analogous experiments to other glycosylation systems should shed further light on their mechanisms and thus assist in the design of better reactions conditions with improved stereoselectivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gabius, H.-J. (ed.) The Sugar Code (Wiley-VCH, 2009).
Martin McGowan, E. D. & Bowman, K. Background Paper on Glycosciences and Glycomics in the United States (National Research Council, 2010).
Wu, C.-Y. & Wong, C.-H. Chemistry and glycobiology. Chem. Commun. 47, 6201–6207 (2011).
Seeberger, P. H. Chemical glycobiology: why now? Nature Chem. Biol. 5, 368–372 (2009).
Boltje, T. J., Buskas, T. & Boons, G.-J. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nature Chem. 1, 611–622 (2009).
Galan, M. C., Benito-Alifonsoa, D. & Watt, G. M. Carbohydrate chemistry in drug discovery. Org. Biomol. Chem. 9, 3598–3610.
Chlubnova, I. et al. Natural glycans and glycoconjugates as immunomodulating agents. Nat. Prod. Rep. 28, 937–952 (2011).
Stallforth, P., Lepenies, B., Adibekian, A. & Seeberger, P. H. Carbohydrates: a frontier in medicinal chemistry. J. Med. Chem. 52, 5561–5576 (2009).
Zhu, X. & Schmidt, R. R. New principles for glycoside-bond formation. Angew. Chem. Int. Ed. 48, 1900–1934 (2009).
Demchenko, A. V. (ed.) Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance (Wiley-VCH, 2008).
Horenstein, N. A. Mechanism for nucleophilic aliphatic substitution at glycosides. Adv. Phys. Org. Chem. 41, 275–314 (2006).
Sinnott, M. L. Carbohydrate Chemistry and Biochemistry (RSC Publishing, 2007).
Barresi, F. & Hindsgaul, O. Chemically synthesized oligosaccharides, 1994. A searchable table of glycosidic linkages. J. Carbohydr. Chem. 14, 1043–1087 (1995).
Bohé, L. & Crich, D. A propos of glycosyl cations and the mechanism of chemical glycosylation. C. R. Chimie 14, 3–16 (2011).
Smith, D. M. & Woerpel, K. A. Electrostatic interactions in cations and their importance in biology and chemistry. Org. Biomol. Chem. 4, 1195–1201 (2006).
Whitfield, D. M. Computational studies of the role of glycopyranosyl oxacarbenium ions in glycobiology and glycochemistry. Adv. Carbohydr. Chem. Biochem. 62, 83–159 (2009).
Satoh, H., Hansen, H. S., Manabe, S., van Gunsteren, W. F. & Hunenberger, P. H. Theoretical investigation of solvent effects on glycosylation reactions: stereoselectivity controlled by preferential conformations of the intermediate oxacarbenium–counterion complex. J. Chem. Theor. Comput. 6, 1783–1797 (2010).
Li, Z. Computational study of the influence of cyclic protecting groups in stereoselectivity of glycosylation reactions. Carbohydr. Res. 345, 1952–1957 (2010).
Saito, K. et al. Indirect cation-flow method: flash generation of alkoxycarbenium ions and studies on the stability of glycosyl cations. Angew. Chem. Int. Ed. 50, 5153–5156 (2011).
Crich, D. & Sun, S. Direct synthesis of β-mannopyranosides by the sulfoxide method. J. Org. Chem. 62, 1198–1199 (1997).
Crich, D. Mechanism of a chemical glycosylation. Acc. Chem. Res. 43, 1144–1153 (2010).
Aubry, S., Sasaki, K., Sharma, I. & Crich, D. Influence of protecting groups on the reactivity and selectivity of glycosylation: chemistry of the 4,6-O-benzylidene protected mannopyranosyl donors and related species. Top. Curr. Chem. 301, 141–188 (2011).
Crich, D. & Sun, S. Are glycosyl triflates intermediates in the sulfoxide glycosylation method? A chemical and 1H, 13C, and 19F-NMR spectroscopic investigation. J. Am. Chem. Soc. 119, 11217–11223 (1997).
Crich, D. Chemistry of glycosyl triflates: synthesis of β-mannopyranosides. J. Carbohydr. Chem. 21, 663–686 (2002).
Berti, P. J. & Tanaka, K. S. E. Transition state analysis using multiple kinetic isotope effects: mechanisms of enzymatic and non-enzymatic glycoside hydrolysis and transfer. Adv. Phys. Org. Chem. 37, 239–313 (2002).
Chan, J., Lewis, A. R., Gilbert, M., Karwaski, M.-F. & Bennet, A. J. A direct NMR method for the measurement of competitive kinetic isotope effects. Nature Chem. Biol. 6, 405–407 (2010).
Melander, L. & Saunders, W. H. Reaction Rates of Isotopic Molecules (Wiley-Interscience, 1980).
Singleton, D. A. & Szymanski, M. J. Simultaneous determination of intermolecular and intramolecular 13C and 2H kinetic isotope effects at natural abundance. J. Am. Chem. Soc. 121, 9455–9456 (1999).
Lee, J. K., Bain, A. D. & Berti, P. J. Probing the transition states of four glucoside hydrolyses with 13C kinetic isotope effects measured at natural abundance by NMR spectroscopy. J. Am. Chem. Soc. 126, 3769–3776 (2004).
Crich, D. & Chandrasekera, N. S. Mechanism of 4,6-O-benzylidene directed β-mannosylation as determined by α-deuterium kinetic isotope effects. Angew. Chem. Int. Ed. 43, 5386–5389 (2004).
Crich, D., Smith, M., Yao, Q. & Picione, J. 2,4,6-Tri-tert-butylpyrimidine (TTBP): a cost effective, readily available alternative to the hindered base 2,6-di-tert-butylpyridine and its 4-substituted derivatives in glycosylation and other reactions. Synthesis 323–326 (2001).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988).
Becke, A. D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Bigeleisen, J., Mayer, M. G. Calculation of equilibrium constants for isotopic exchange reactions. J. Chem. Phys. 15, 261–267 (1947).
Seeman, J. I. Effect of conformational change on reactivity in organic chemistry. Chem. Rev. 83, 83–134 (1983).
Lemieux, R. U. in Molecular Rearrangements (ed. De Mayo, P.) Part 2, 709–769 (Interscience Publishers, 1964).
Walvoort, M. T. C. et al. Equatorial anomeric triflates from mannuronic acid esters. J. Am. Chem. Soc. 131, 12080–12081 (2009).
Crich, D. & Sharma, I. Is donor–acceptor hydrogen bonding necessary for 4,6-O-benzylidene-directed β-mannopyranosylation? Stereoselective synthesis of β-C-mannopyranosides and α-C-glucopyranosides. Org. Lett. 10, 4731–4734 (2008).
Crich, D. & Sharma, I. Influence of the O3 protecting group on stereoselectivity in the preparation of C-mannopyranosides with 4,6-O-benzylidene protected donors. J. Org. Chem. 75, 8383–8391 (2010).
Frisch, M. et al. Gaussian 09 (Gaussian, 2010).
Acknowledgements
The authors acknowledge the Natural Sciences and Engineering Research Council (NSERC) of Canada and the National Institutes of Health (NIH, GM62160) for grant support to D.A.P. and D.C., respectively. M.H. and G.E.G. thank the Ministère de l'Education Nationale de la Recherche et de la Technologie and NSERC, respectively, for scholarships. D.A.P. acknowledges support from the Canada Research Chairs programme.
Author information
Authors and Affiliations
Contributions
L.B., D.A.P. and D.C. designed the project and wrote the manuscript. M.H. carried out the synthetic work. G.E.G. conducted the computational studies. N.B. and M.H. performed the NMR study. M.H., N.B., L.B. and D.C. analysed the NMR studies, and G.E.G. and D.A.P. analysed the computational work. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1125 kb)
Rights and permissions
About this article
Cite this article
Huang, M., Garrett, G., Birlirakis, N. et al. Dissecting the mechanisms of a class of chemical glycosylation using primary 13C kinetic isotope effects. Nature Chem 4, 663–667 (2012). https://doi.org/10.1038/nchem.1404
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1404
This article is cited by
-
Light Scattering in Non-aqueous Solutions of Low-Molecular-Mass Compounds: Application for Supramer Analysis of Reaction Solutions
Journal of Solution Chemistry (2020)
-
Stereoselective oxidative glycosylation of anomeric nucleophiles with alcohols and carboxylic acids
Nature Communications (2018)
-
Glycosyl Sulfoxides in Glycosylation Reactions
Topics in Current Chemistry (2018)
-
A minimalist approach to stereoselective glycosylation with unprotected donors
Nature Communications (2017)
-
Recent progress on the synthesis of 2-deoxy glycosides
Science China Chemistry (2017)