Abstract
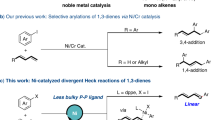
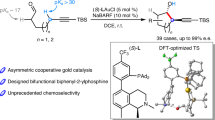
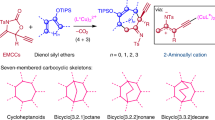
Since the discovery of the Cope rearrangement in the 1940s, no asymmetric variant of the rearrangement of achiral 1,5-dienes has emerged, despite the successes that have been achieved with its heteroatom variants (Claisen, aza-Cope, and so on). This article reports the first example of an enantioselective Cope reaction that starts from an achiral diene. The new gold(I) catalyst derived from double Cl−-abstraction of ((S)-3,5-xylyl-PHANEPHOS(AuCl)2), has been developed for the sigmatropic rearrangement of alkenyl-methylenecyclopropanes. The reaction proceeds at low temperature and the synthetically useful vinylcyclopropane products are obtained in high yield and enantioselectivity. Density functional theory calculations predict that: (1) the reaction proceeds via a cyclic carbenium ion intermediate, (2) the relief of strain in the methylenecyclopropane moiety provides the thermodynamic driving force for the rearrangement and (3) metal complexation of the transition-state structure lowers the rearrangement barriers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cope, A. C. & Hardy, E. M. The introduction of substituted vinyl groups. V. A rearrangement involving the migration of an allyl group in a three-carbon system. J. Am. Chem. Soc. 62, 441–444 (1940).
Nubbemeyer, U. Recent advances in asymmetric [3,3]-sigmatropic rearrangements. Synthesis 961–1008 (2003).
Allin, S. M. & Baird, R. D. Development and synthetic applications of asymmetric [3,3]-sigmatropic rearrangements. Curr. Org. Chem. 5, 395–415 (2001).
Watson, M. P., Overman, L. E. & Bergman, R. G. Kinetic and computational analysis of the palladium(II)-catalyzed asymmetric allylic trichloroacetimidate rearrangement: development of a model for enantioselectivity. J. Am. Chem. Soc. 129, 5031–5044 (2007).
Hansen, J. H. et al. On the mechanism and selectivity of the combined C–H activation/Cope rearrangement. J. Am. Chem. Soc. 133, 5076–5085 (2011).
Lian, Y. & Davies, H. M. L. Combined C–H functionalization/Cope rearrangement with vinyl ethers as a surrogate for the vinylogous Mukaiyama aldol reaction. J. Am. Chem. Soc. 133, 11940–11943 (2011).
Hoffmann, R. & Stohrer, W-D. Cope rearrangement revisited. J. Am. Chem. Soc. 93, 6941–6948 (1971).
Wendt, K. U., Schulz, G. E. & Corey, E. J. Enzyme mechanisms for polycyclic triterpene formation. Angew. Chem. Int. Ed. 39, 2812–2833 (2000).
Yoder, R. A. & Johnston, J. N. A case study in biomimetic total synthesis: polyolefin carbocyclizations to terpenes and steroids. Chem. Rev. 105, 4730–4756 (2005).
Rubin, M., Rubina, M. & Gevorgyan, V. Transition metal chemistry of cyclopropenes and cyclopropanes. Chem. Rev. 107, 3117–3179 (2007).
Lu, B-L., Dai, L. & Shi, M. Strained small rings in gold-catalyzed rapid chemical transformations. Chem. Soc. Rev. doi: 10.1039/C2CS15295A (2012).
Seiser, T. & Cramer, N. Enantioselective metal-catalyzed activation of strained rings. Org. Biomol. Chem. 7, 2835–2840 (2009).
Leemans, E., D'hooghe, M. & De Kimpe, N. Ring expansion of cyclobutylmethylcarbenium ions to cyclopentane or cyclopentene derivatives and metal-promoted analogous rearrangements. Chem. Rev. 111, 3268–3333 (2011).
Fürstner, A. & Aïssa, C. PtCl2-catalyzed rearrangement of methylenecyclopropanes. J. Am. Chem. Soc. 128, 6306–6307 (2006).
Seiser, T., Saget, T., Tran, D. N. & Cramer, N. Cyclobutanes in catalysis. Angew. Chem. Int. Ed. 50, 7740–7752 (2011).
Mauleόn, P., Krinsky, J. L. & Toste, F. D. Mechanistic studies on Au(I)-catalyzed [3,3]-sigmatropic rearrangements using cyclopropane probes. J. Am. Chem. Soc. 131, 4513–4520 (2009).
Frisch, M. J. et al. GAUSSIAN09, Revision A. 02; Gaussian, Inc., Wallingford CT, 2009.
Sokol, J. G., Korapala, C. S., White, P. S., Becker, J. J. & Gagné, M. R. Terminating platinum-initiated cation–olefin reactions with simple alkenes. Angew. Chem. Int. Ed. 50, 5658–5661 (2011).
Sethofer, S. G., Meyer, T. & Toste, F. D. Gold(I)-catalyzed enantioselective polycyclization reactions. J. Am. Chem. Soc. 132, 8276–8277 (2010).
Pradal, A., Chao, C-M., Vitale, M. R., Toullec, P. Y. & Michelet, V. Asymmetric Au-catalyzed domino cyclization/nucleophile addition reactions of enynes in the presence of water, methanol and electron-rich aromatic derivatives. Tetrahedron 67, 4371–4377 (2011).
Mézailles, N., Ricard, L. & Gagosz, F. Phosphine gold(I) bis-(trifluoromethanesulfonyl)imidate complexes as new highly efficient and air-stable catalysts for the cycloisomerization of enynes. Org. Lett. 7, 4133–4136 (2005).
Overman, L. E. & Renaldo, A. F. Mechanism of the palladium dichloride catalyzed Cope rearrangement of acyclic dienes. A substituent effect study. J. Am. Chem. Soc. 112, 3945–3949 (1990).
Nakamura, H., Iwama, H., Ito, M. & Yamamoto, Y. Palladium(0)-catalyzed Cope rearrangement of acyclic 1,5-dienes. Bis(π-allyl)palladium(II) intermediate. J. Am. Chem. Soc. 121, 10850–10851 (1999).
Fanning, K. N., Jamieson, A. G. & Sutherland, A. Palladium(II)-catalyzed rearrangement reactions. Curr. Org. Chem. 10, 1007–1020 (2006).
Siebert, M. R. & Tantillo, D. J. Transition-state complexation in palladium-promoted [3,3] sigmatropic shifts. J. Am. Chem. Soc. 129, 8686–8687 (2007).
Nowicki, J. Claisen, Cope and related rearrangements in the synthesis of flavour and fragrance compounds. Molecules 5, 1033–1050 (2000).
Blechert, S. The hetero-Cope rearrangement in organic synthesis. Synthesis 71–82 (1989).
Koh, J. H., Mascarenhas, C. & Gagné, M. R. Pd(II)-catalyzed cyclogeneration of carbocations: subsequent rearrangement and trapping under oxidative conditions. Tetrahedron 60, 7405–7410 (2004).
Korotchenko, V. N. & Gagné, M. R. Palladium-catalyzed cyclization of 1,ω-dienols: multiple ways to intramolecularly trap a carbocation. J. Org. Chem. 72, 4877–4881 (2007).
Fürstner, A. Gold and platinum catalysis – a convenient tool for generating molecular complexity. Chem. Soc. Rev. 38, 3208–3221 (2009).
Li, Z., Brouwer, C. & He. C. Gold-catalyzed organic transformations. Chem. Rev. 108, 3239–3265 (2008).
Gorin, D. J., Sherry, B. D. & Toste, F. D. Ligand effects in homogeneous Au catalysis. Chem. Rev. 108, 3351–3378 (2008).
Widenhoefer, R. A. Recent developments in enantioselective gold(I) catalysis. Chem. Eur. J. 14, 5382–5391 (2008).
Fürstner, A. & Davies, P. W. Catalytic carbophilic activation: catalysis by platinum and gold π acids. Angew. Chem. Int. Ed. 46, 3410–3449 (2007).
Hashmi, A. S. K. The catalysis gold rush: new claims. Angew. Chem. Int. Ed. 44, 6990–6993 (2005).
Kleinbeck, F. & Toste, F. D. Gold(I)-catalyzed enantioselective ring expansion of allenylcyclopropanols. J. Am. Chem. Soc. 131, 9178–9179 (2009).
Hamilton, G. L., Kang, E. J., Mba, M. & Toste, F. D. A powerful chiral counterion strategy for asymmetric transition metal catalysis. Science 317, 496–499 (2007).
Tarselli, M. A., Chianese, A. R., Lee, S. J. & Gagné, M. R. Gold(I)-catalyzed asymmetric cycloisomerization of eneallenes into vinylcyclohexenes. Angew. Chem. Int. Ed. 46, 6670–6673 (2007).
Stafford, J. A. & McMurry, J. E. An efficient method for the preparation of alkylidenecyclopropanes. Tetrahedron Lett. 29, 2531–2534 (1988).
Sethofer, S. G., Staben, S. T., Hung, O. T. & Toste, F. D. Au(I)-catalyzed ring expanding cycloisomerization: total synthesis of ventricosene. Org. Lett. 10, 4315–4318 (2008).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Zhao, Y. & Truhlar, D. G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 41, 157–167 (2008).
Barone, V. & Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998).
Johnson, W. T. G. & Borden, W. T. Why are methylenecyclopropane and 1-methylcyclopropene more ‘strained’ than methylcyclopropane? J. Am. Chem. Soc. 119, 5930–5933 (1997).
Bach, R. D. & Dmitrenko, O. Strain energy of small ring hydrocarbons. Influence of C–H bond dissociation energies. J. Am. Chem. Soc. 126, 4444–4452 (2004).
Acknowledgements
The University of North Carolina group acknowledges the National Institute of General Medicine (GM-60578), D.W. acknowledges the Fulbright Foreign Student Program, D.J.T. acknowledges support from the ACS-PRF program (49119-ND4) and the National Science Foundation's Partnership for Advanced Computational Infrastructure (CHE-030089, Pittsburgh Supercomputer Center) and O.G. acknowledges R.M. Hussing, and M. and L. Defenbaugh for support.
Author information
Authors and Affiliations
Contributions
R.J.F., D.W. and M.R.G. conceived and designed the experiments. R.J.F. performed the experiments and analysed the data. R.J.F., D.J.T. and M.R.G. co-wrote the paper. O.G. and D.J.T. performed the DFT calculations. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Crystallographic data for compound 4. (CIF 24 kb)
Rights and permissions
About this article
Cite this article
Felix, R., Weber, D., Gutierrez, O. et al. A gold-catalysed enantioselective Cope rearrangement of achiral 1,5-dienes. Nature Chem 4, 405–409 (2012). https://doi.org/10.1038/nchem.1327
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1327
This article is cited by
-
Zwitterionic Bergman cyclization triggered polymerization gives access to metal-graphene nanoribbons using a boron metal couple
Communications Chemistry (2023)
-
Photocatalyzed [2+1] cyclization of alkenes and silylated trifluorodiazoethanes: facile entry into (difluoromethylene)cyclopropanes
Science China Chemistry (2023)
-
Computational study of homogenous gold-catalyzed oxime–oxime rearrangement: Balci–Güven rearrangement
Structural Chemistry (2020)