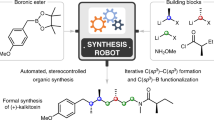
Abstract
Fluorous mixture synthesis minimizes the effort to synthesize small-molecule libraries by labelling the molecules rather than the reaction vessels. Reactants are labelled with fluorinated tags and products can later be demixed based on the fluorine content. A limit in the number of available tags can be overcome by using binary encoding so that a total of four tags can label uniquely a library of 16 compounds. This strategy, however, means that separation based on fluorine content alone is not possible. Here, we solve this problem by selectively removing one tag after an initial demixing step; a second demixing provides each individual compound. The usefulness of this strategy is demonstrated by the synthesis of a library that contains all 16 diastereomers of the natural products macrosphelides A and E. Macrosphelide D was not in this library, and so its assigned structure was incorrect. We determined its constitution by using NMR spectroscopy and its configuration by synthesizing four candidate stereoisomers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
18 January 2012
In the version of this Article originally published, it erroneously stated the authors had no competing financial interests. This information has now been corrected in the HTML and PDF versions of the Article.
References
Luo, Z. Y., Zhang, Q. S., Oderaotoshi, Y. & Curran, D. P. Fluorous mixture synthesis: a fluorous-tagging strategy for the synthesis and separation of mixtures of organic compounds. Science 291, 1766–1769 (2001).
Zhang, W. Fluorous mixture synthesis (FMS) of enantiomers, diastereomers, and compound libraries. Arkivoc 101–109 (2004).
Curran, D. P. in The Handbook of Fluorous Chemistry (eds Gladysz, J. A., Curran, D. P. & Horvath, I. T.) 128–156 (Wiley-VCH, 2004).
Zhang, Q. S. & Curran, D. P. Quasienantiomers and quasiracemates: new tools for identification, analysis, separation, and synthesis of enantiomers. Chem. Eur. J. 11, 4866–4880 (2005).
O'Brien, M., Denton, R. & Ley, S. V. Lesser-known enabling technologies for organic synthesis. Synthesis 1157–1192 (2011).
Bejot, R. et al. Fluorous synthesis of 18F radiotracers with the [18F]fluoride ion: nucleophilic fluorination as the detagging process. Angew. Chem. Int. Ed. 48, 586–589 (2009).
McAllister, L. A. et al. A fluorous, Pummerer cyclative-capture strategy for the synthesis of N-heterocycles. Chem. Eur. J. 13, 1032–1046 (2007).
Yoshida, J. & Itami, K. Tag strategy for separation and recovery. Chem. Rev. 102, 3693–3716 (2002).
Curran, D. P. Strategy-level separations in organic synthesis: from planning to practice. Angew. Chem. Int. Ed. 37, 1175–1196 (1998).
Dandapani, S., Jeske, M. & Curran, D. P. Synthesis of all 16 stereoisomers of pinesaw fly sex pheromones – tools and tactics for solving problems in fluorous mixture synthesis. J. Org. Chem. 70, 9447–9462 (2005).
Dandapani, S., Jeske, M. & Curran, D. P. Stereoisomer libraries: total synthesis of all 16 stereoisomers of the pine sawfly sex pheromone by a fluorous mixture-synthesis approach. Proc. Natl Acad. Sci. USA 101, 12008–12012 (2004).
Curran, D. P. in The Handbook of Fluorous Chemistry (eds Gladysz, J. A., Curran, D. P. & Horvath, I. T.) 101–127 (Wiley-VCH, 2004).
Yang, F., Newsome, J. J. & Curran, D. P. Structure assignment of lagunapyrone B by fluorous mixture synthesis of four candidate stereoisomers. J. Am. Chem. Soc. 128, 14200–14205 (2006).
Jung, W-H. et al. Confirmation of the stereostructure of (+)-cytostatin by fluorous mixture synthesis of four candidate stereoisomers. Angew. Chem. Int. Ed. 47, 1130–1133 (2008).
Curran, D. P., Moura-Letts, G. & Pohlman, M. Solution-phase mixture synthesis with fluorous tagging en route: total synthesis of an eight-member stereoisomer library of passifloricins. Angew. Chem. Int. Ed. 45, 2423–2426 (2006).
Wilcox, C. S. & Turkyilmaz, S. Oligomeric ethylene glycols as sorting tags for parallel and combinatorial mixture synthesis. Tetrahedron Lett. 46, 1827–1829 (2005).
Gudipati, V., Curran, D. P. & Wilcox, C. S. Solution-phase parallel synthesis with oligoethylene glycol sorting tags. Preparation of all four stereoisomers of the hydroxybutenolide fragment of murisolin and related acetogenins. J. Org. Chem. 71, 3599–3607 (2006).
Curran, D. P. et al. Total synthesis of a 28-member stereoisomer library of murisolins. J. Am. Chem. Soc. 128, 9561–9573 (2006).
Hayashi, M. et al. Macrosphelide, a novel inhibitor of cell–cell adhesion molecule. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 48, 1435–1439 (1995).
Sunazuka, T. et al. Relative and absolute stereochemistries and total synthesis of (+)-macrosphelides A and B, potent, orally bioavailable inhibitors of cell–cell adhesion. J. Am. Chem. Soc. 119, 10247–10248 (1997).
Sunazuka, T. et al. Absolute stereochemistries and total synthesis of (+)/(−)-macrosphelides, potent, orally bioavailable inhibitors of cell–cell adhesion. Tetrahedron 61, 3789–3803 (2005).
Numata, A. et al. Novel antitumour metabolites produced by a fungal strain from a sea hare. Tetrahedron Lett. 38, 8215–8218 (1997).
Yamada, T. et al. Absolute stereostructures of cell-adhesion inhibitors, macrosphelides C, E–G and I, produced by a Periconia species separated from an Aplysia sea hare. J. Chem. Soc. Perkin Trans. 1 3046–3053 (2001).
Takamatsu, S. et al. Macrosphelides C and D, novel inhibitors of cell adhesion. J. Antibiot. 50, 878–880 (1997).
Schelhaas, M. & Waldmann, H. Protecting group strategies in organic synthesis. Angew. Chem. Int. Ed. Engl. 35, 2056–2083 (1996).
Greene, T. W. & Wuts, P. G. M. Protective Groups in Organic Synthesis 3rd edn (Wiley-Interscience, 1999).
Kocienski, P. J. Protecting Groups (Thieme, 1999).
Matsuya, Y. & Nemoto, H. Recent advances in macrosphelide synthesis. Heterocycles 65, 1741–1749 (2005).
Takahashi, T., Kusaka, S., Doi, T., Sunazuka, T. & Omura, S. A combinatorial synthesis of a macrosphelide library utilizing a palladium-catalyzed carbonylation on a polymer support. Angew. Chem. Int. Ed. 42, 5230–5234 (2003).
Prasad, K. R. & Gutala, P. Enantioselective total synthesis of macrosphelides A and E. Tetrahedron 67, 4514–4520 (2011).
Paek, S-M. et al. Concise syntheses of (+)-macrosphelides A and B. Org. Lett. 7, 3159–3162 (2005).
Inanaga, J., Hirata, K., Hiroko, S., Katsuki, T. J. & Yamaguchi, M. A rapid esterification by means of mixed anhydride and its application to large-ring lactonization. Bull. Chem. Soc. Jpn 52, 1989–1993 (1979).
Yamada, T., Minoura, K., Tanaka, R. & Numata, A. Cell-adhesion inhibitors produced by a sea hare-derived Periconia sp. J. Antibiot. 60, 370–375 (2007).
Corey, E. J., Brunelle, D. J. & Nicolaou, K. C. A translactonization route to macrocyclic lactones. J. Am. Chem. Soc. 99, 7359–7360 (1977).
Wender, P. A., Gulledge, A. V., Jankowski, O. D. & Seto, H. Isoapoptolidin: a structure and activity of the ring-expanded isomer of apoptolidin. Org. Lett. 4, 3819–3822 (2002).
Pennington, J. D., Williams, H. J., Salomon, A. R. & Sulikowski, G. A. Toward a stable apoptolidin derivative: identification of isoapoptolidin and selective deglycosylation of apoptolidin. Org. Lett. 4, 3823–3825 (2002).
Park, P. K., O'Malley, S. J., Schmidt, D. R. & Leighton, J. L. Total synthesis of dolabelide D. J. Am. Chem. Soc. 128, 2796–2797 (2006).
Jung, W. H. et al. Total synthesis and biological evaluation of C16 analogs of (–)-dictyostatin. J. Med. Chem. 50, 2951–2966 (2007).
Acknowledgements
We thank the National Institutes of Health for research support (National Institute of General Medical Sciences) and for a grant to purchase a 700 MHz NMR spectrometer. D-H.C. was supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2005-214-C00214). We thank K. Damodaran and S. Bowser for help with NMR spectra. We thank T. Yamada, Osaka University of Pharmaceutical Sciences, for providing copies of the spectra of macrosphelide M.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the experiments and analysed the data. All authors, except D.P.C., performed the experiments: D-H.C. made the precursors and prepared a preliminary library, J.J.S. made the final library and carried out the demixing steps with K.Z., K.Z. purified and characterized the library members and M.K.S. made and characterized the macrosphelide D candidates. D.P.C wrote the paper. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.P.C. owns an equity interest in Fluorous Technologies, Inc., supplier of some reagents and HPLC columns used in this work. The other authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 8449 kb)
Supplementary information
Chemical compounds table 1 (XLS 20 kb)
Supplementary information
Chemical compounds table 2 (XLS 32 kb)
Rights and permissions
About this article
Cite this article
Curran, D., Sinha, M., Zhang, K. et al. Binary fluorous tagging enables the synthesis and separation of a 16-stereoisomer library of macrosphelides. Nature Chem 4, 124–129 (2012). https://doi.org/10.1038/nchem.1233
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1233
This article is cited by
-
Synergy of synthesis, computation and NMR reveals correct baulamycin structures
Nature (2017)
-
Recent progress on fluorous synthesis of biologically interesting compounds
Molecular Diversity (2014)