Abstract
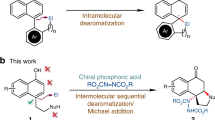
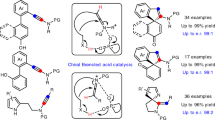
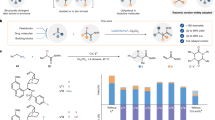
Successful implementation of a catalytic asymmetric synthesis strategy to produce enantiomerically enriched compounds requires the adoption of suitable prochiral substrates. The combination of an azomethine imine electrophile with various nucleophiles could give straightforward access to a number of synthetically useful chiral hydrazines, but is used rarely. Here we report the exploitation of acyclic azomethine imines as a new type of prochiral electrophile. They can be generated in situ by the condensation of N′-benzylbenzoylhydrazide with a variety of aldehydes in the presence of a catalytic amount of an axially chiral dicarboxylic acid. By trapping these electrophiles with alkyl diazoacetate or (diazomethyl)phosphonate nucleophiles, we produced a diverse array of chiral α-diazo-β-hydrazino esters and phosphonates with excellent enantioselectivities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Miller, E. D., Kauffman, C. A., Jensen, P. R. & Fenical, W. Piperazimycins: cytotoxic hexadepsipeptides from a marine-derived bacterium of the genus Streptomyces. J. Org. Chem. 72, 323–330 (2006).
Li, W., Gan, J. & Ma, D. Total synthesis of piperazimycin A: a cytotoxic cyclic hexadepsipeptide. Angew. Chem. Int. Ed. 48, 8891–8895 (2009).
Bold, G. et al. New aza-dipeptide analogues as potent and orally absorbed HIV-1 protease inhibitors: candidates for clinical development. J. Med. Chem. 41, 3387–3401 (1998).
Pyrko, P. et al. HIV-1 protease inhibitors nelfinavir and atazanavir induce malignant glioma death by triggering endoplasmic reticulum stress. Cancer Res. 67, 10920–10928 (2007).
Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 30, 205–213 (2001).
Nair, V., Biju, A. T., Mathew, S. C. & Babu, B. P. Carbon–nitrogen bond-forming reactions of dialkyl azodicarboxylate: a promising synthetic strategy. Chem. Asian J. 3, 810–820 (2008).
Küchenthal, C-H. & Maison, W. Synthesis of cyclic hydrazino α-carboxylic acids. Synthesis 2010, 719–740 (2010).
Friedstad, G. K. Chiral N-acylhydrazones: versatile imino acceptors for asymmetric amine synthesis. Eur. J. Org. Chem. 2005, 3157–3172 (2005).
Sugiura, M. & Kobayashi, S. N-Acylhydrazones as versatile electrophiles for the synthesis of nitrogen-containing compounds. Angew. Chem. Int. Ed. 44, 5176–5186 (2005).
Denmark, S. E. & Nicaise, O. J-C. in Comprehensive Asymmetric Catalysis Vol. 2 (eds Jacobsen, E. N., Pfaltz, A. & Yamamoto, H.) Ch. 26.2 (Springer, 1999).
Hashimoto, T. & Maruoka, K. in Handbook of Cyclization Reactions Vol. 1 (ed. Ma, S.) Ch. 3 (Wiley, 2009).
Pellissier, H. Asymmetric 1,3-dipolar cycloadditions. Tetrahedron 63, 3235–3285 (2007).
Stanley, L. M. & Sibi, M. P. Enantioselective copper-catalyzed 1,3-dipolar cycloadditions. Chem. Rev. 108, 2887–2902 (2008).
Kawai, H., Kusuda, A., Nakamura, S., Shiro, M. & Shibata, N. Catalytic enantioselective trifluoromethylation of azomethine imines with trimethyl(trifluoromethyl)silane. Angew. Chem. Int. Ed. 48, 6324–6327 (2009).
Shintani, R., Soh, Y-T. & Hayashi, T. Rhodium-catalyzed asymmetric arylation of azomethine imines. Org. Lett. 12, 4106–4109 (2010).
Hashimoto, T., Maeda, Y., Omote, M., Nakatsu, H. & Maruoka, K. Catalytic enantioselective 1,3-dipolar cycloaddition of C,N-cyclic azomethine imines with α,β-unsaturated aldehydes. J. Am. Chem. Soc. 132, 4076–4077 (2010).
Hashimoto, T., Omote, M. & Maruoka, K. Asymmetric inverse-electron-demand 1,3-dipolar cycloaddition of C,N-cyclic azomethine imines: an umpolung strategy. Angew. Chem. Int. Ed. 50, 3489–3492 (2011).
Oppolzer, W. Ein neuer, flexibler augang zu pyrazolidinen und pyrazolinen. Tetrahedron Lett. 11, 2199–2204 (1970).
Oppolzer, W. Intramolekulare cycloadditionen von azomethiniminen, teil I: reaktion von ungesaettigten aldehyden mit N-acyl-N ′-alkylhydraziden. Tetrahedron Lett. 11, 3091–3094 (1970).
Oppolzer, W. Intramolekulare cycloadditionen von azomethiniminen, teil II: reaktionen von ungesaettigten hydraziden mit aldehyden. Tetrahedron Lett. 13, 1707–1710 (1972).
Oppolzer, W. & Peter Weber, H. Die thermolyse von quecksilber-bis (N,N-dimethyl-N ′-phenacetylhydrazin) in gegenwart dipolarophiler olefine. Tetrahedron Lett. 13, 1711–1714 (1972).
Jacobi, P. A., Brownstein, A., Martinelli, M. & Grozinger, K. A mild procedure for the generation of azomethine imines. Stereochemical factors in the intramolecular 1,3-dipolar addition of azomethine imines and a synthetic approach to saxitoxin. J. Am. Chem. Soc. 103, 239–241 (1981).
Jacobi, P. A., Martinelli, M. J. & Polanc, S. Total synthesis of (±)-saxitoxin. J. Am. Chem. Soc. 106, 5594–5598 (1984).
Nilsson, B. L., Overman, L. E., Read de Alaniz, J. & Rohde, J. M. Enantioselective total syntheses of nankakurines A and B: confirmation of structure and establishment of absolute configuration. J. Am. Chem. Soc. 130, 11297–11299 (2008).
Kanemasa, S., Tomoshige, N., Wada, E. & Tsuge, O. Triethylamine-mediated generation of a synthetic equivalent of parent azomethine imine by condensation of ethyl 3-benzylcarbazate with paraformaldehyde. Bull. Chem. Soc. Jpn 62, 3944–3949 (1989).
Tamura, Y., Minamikawa, J-I., Miki, Y., Okamoto, Y. & Ikeda, M. The synthesis and properties of N-acylimino-3,4-dihydroisoquinolinium betaines. Yakugaku Zasshi 93, 648 (1973).
Portlock, D. E., Naskar, D., West, L. & Li, M. Petasis boronic acid–Mannich reactions of substituted hydrazines: synthesis of α-hydrazinocarboxylic acids. Tetrahedron Lett. 43, 6845–6847 (2002).
Akiyama, T. Stronger Brønsted acids. Chem. Rev. 107, 5744–5758 (2007).
Terada, M. Chiral phosphoric acids as versatile catalysts for enantioselective transformations. Synthesis 2010, 1929–1982 (2010).
Hashimoto, T. & Maruoka, K. Design of axially chiral dicarboxylic acid for asymmetric Mannich reaction of arylaldehyde N-Boc imines and diazo compounds. J. Am. Chem. Soc. 129, 10054–10055 (2007).
Hashimoto, T. & Maruoka, K. Design of an axially chiral dicarboxylic acid and its application in syntheses of optically active β-amino acids and β-amino phosphonic acid derivatives. Synthesis 2008, 3703–3706 (2008).
Hashimoto, T., Hirose, M. & Maruoka, K. Asymmetric imino aza-enamine reaction catalyzed by axially chiral dicarboxylic acid: use of arylaldehyde N,N-dialkylhydrazones as acyl anion equivalent. J. Am. Chem. Soc. 130, 7556–7557 (2008).
Hashimoto, T., Uchiyama, N. & Maruoka, K. Trans-selective asymmetric aziridination of diazoacetamides and N-Boc imines catalyzed by axially chiral dicarboxylic acid. J. Am. Chem. Soc. 130, 14380–14381 (2008).
Hashimoto, T., Kimura, H. & Maruoka, K. Enantioselective formal alkenylations of imines catalyzed by axially chiral dicarboxylic acid using vinylogous aza-enamines. Angew. Chem. Int. Ed. 49, 6844–6847 (2010).
Ramón, D. J. & Yus, M. Asymmetric multicomponent reactions (AMCRs): the new frontier. Angew. Chem. Int. Ed. 44, 1602–1634 (2005).
Uraguchi, D., Sorimachi, K. & Terada, M. Organocatalytic asymmetric direct alkylation of α-diazoester via C−H bond cleavage. J. Am. Chem. Soc. 127, 9360–9361 (2005).
Maruoka, K., Itoh, T., Shirasaka, T. & Yamamoto, H. Asymmetric hetero-Diels–Alder reaction catalyzed by a chiral organoaluminum reagent. J. Am. Chem. Soc. 110, 310–312 (1988).
Hashimoto, T., Takagaki, T., Kimura, H. & Maruoka, K. Modular synthesis of axially chiral 3,3′-disilyl dicarboxylic acids by silalactones. Chem. Asian J. DOI: 10.1002/asia.201100172.
Zelenin, K. N. et al. Synthesis of 5-hydroxy- and 5-acylhydrazinopyrazolidines by the reaction of β-substituted hydrazides with α,β-unsaturated aldehydes and their biological activity. Chem. Heterocycl. Compd 20, 529–536 (1984).
Tanaka, K., Kato, T., Fujinami, S., Ukaji, Y. & Inomata, K. Asymmetric 1,3-dipolar cycloaddition of azomethine imines to homoallylic alcohols. Chem. Lett. 39, 1036–1038 (2010).
Palacios, F., Alonso, C. & de los Santos, J. M. Synthesis of β-aminophosphonates and -phosphinates. Chem. Rev. 105, 899–932 (2005).
Ding, H. & Friestad, G. K. Trifluoroacetyl-activated nitrogen−nitrogen bond cleavage of hydrazines by samarium(II) iodide. Org. Lett. 6, 637–640 (2004).
Córdova, A. The direct catalytic asymmetric Mannich reaction. Acc. Chem. Res. 37, 102–112 (2004).
Ting, A. & Schaus, S. E. Organocatalytic asymmetric Mannich reactions: new methodology, catalyst design, and synthetic applications. Eur. J. Org. Chem. 5797–5815 (2007).
Verkade, J. M. M., van Hemert, L. J. C., Quaedflieg, P. J. L. M. & Rutjes, F. P. J. T. Organocatalysed asymmetric Mannich reactions. Chem. Soc. Rev. 37, 29–41 (2008).
Kobayashi, S., Mori, Y., Fossey, J. S. & Salter, M. M. Catalytic enantioselective formation of C−C bonds by addition to imines and hydrazones: a ten-year update. Chem. Rev. 111, 2626–2704 (2011).
Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan. T.H. thanks a Grant-in-Aid for Young Scientists (B).
Author information
Authors and Affiliations
Contributions
T.H. conceived the study and wrote the manuscript. H.K. principally performed the experiments. Y.K. assisted preliminary experiments. K.M. organized the research. All authors contributed to designing the experiments, analysing data and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 19785 kb)
Supplementary information
Crystallographic data for compound 7o (CIF 43 kb)
Rights and permissions
About this article
Cite this article
Hashimoto, T., Kimura, H., Kawamata, Y. et al. Generation and exploitation of acyclic azomethine imines in chiral Brønsted acid catalysis. Nature Chem 3, 642–646 (2011). https://doi.org/10.1038/nchem.1096
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1096
This article is cited by
-
Benzenoid-quinoid tautomerism of azomethines and their structural analogs 56. Azomethine imines, derivatives of salicylic and 2-hydroxynaphthoic aldehydes
Russian Chemical Bulletin (2016)
-
New ionochromic azomethinimine chemosensors
Russian Chemical Bulletin (2015)