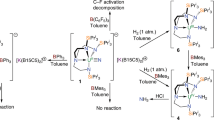
Abstract
Bioinspired hydrogenation of N2 to ammonia at ambient conditions by stepwise nitrogen protonation/reduction with metal complexes in solution has experienced remarkable progress. In contrast, the highly desirable direct hydrogenation with H2 remains difficult. In analogy to the heterogeneously catalysed Haber–Bosch process, such a reaction is conceivable via metal-centred N2 splitting and unprecedented hydrogenolysis of the nitrido ligands to ammonia. We report the synthesis of a ruthenium(IV) nitrido complex. The high nucleophilicity of the nitrido ligand is demonstrated by unusual N–C coupling with π-acidic CO. Furthermore, the terminal nitrido ligand undergoes facile hydrogenolysis with H2 at ambient conditions to produce ammonia in high yield. Kinetic and quantum chemical examinations of this reaction suggest cooperative behaviour of a phosphorus–nitrogen–phosphorus pincer ligand in rate-determining heterolytic hydrogen splitting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Allen, A. D. & Senoff, C. V. Nitrogenopentammineruthenium(II) complexes. J. Chem. Soc. Chem. Commun. 621–622 (1965).
Chatt, J., Dilworth, J. R. & Richards, R. L. Recent advances in the chemistry of nitrogen fixation. Chem. Rev. 78, 589–625 (1978).
Gambarotta, S. Dinitrogen fixation and activation after 30 years: a puzzle still unsolved. J. Organometal. Chem. 500, 117–126 (1995).
Bazhenova, T. A. & Shilov, A. E. Nitrogen fixation in solution. Coord. Chem. Rev. 144, 69–145 (1995).
Hidai, M. & Mizobe, Y. Recent advances in the chemistry of dinitrogen complexes. Chem. Rev. 95, 1115–1133 (1995).
Fryzuk, M. D. & Johnson, S. A. The continuing story of dinitrogen activation. Coord. Chem. Rev. 200–202, 379–409 (2000).
Gambarotta, S. & Scott, J. Multimetallic cooperative activation of N2 . Angew. Chem. Int. Ed. 43, 5298–5308 (2004).
MacKay, B. A. & Fryzuk, M. D. Dinitrogen coordination chemistry: on the biomimetic borderlands. Chem. Rev. 104, 385–402 (2004).
Ohki, Y. & Fryzuk, M. D. Dinitrogen activation by group 4 metal complexes. Angew. Chem. Int. Ed. 46, 3180–3183 (2007).
Schrock, R. R. Catalytic reduction of dinitrogen to ammonia by molybdenum: theory versus experiment. Angew. Chem. Int. Ed. 47, 5512–5522 (2008).
Einsle, O. et al. Nitrogenase MoFe–protein at 1.16 Å resolution: a central ligand in the FeMo–cofactor. Science 297, 1697–1700 (2002).
Yang, T-C. et al. The interstitial atom of the nitrogenase FeMo–cofactor: ENDOR and ESEEM evidence that it is not a nitrogen. J. Am. Chem. Soc. 127, 12804–12805 (2005).
Dance, I. The mechanistically significant coordination chemistry of dinitrogen at FeMo–co, the catalytic site of nitrogenase. J. Am. Chem. Soc. 129, 1076–1088 (2007).
Chatt, J., Pearman, A. J. & Richards, R. L. The reduction of mono-coordinated molecular nitrogen to ammonia in a protic environment. Nature 253, 39–40 (1975).
Nishibayashi, Y., Iwai, S. & Hidai, M. Bimetallic system for nitrogen fixation: ruthenium-assisted protonation of coordinated N2 on tungsten with H2 . Science 279, 540–542 (1998).
Betley, T. A. & Peters, J. C. A tetrahedrally coordinated L3Fe–Nx platform that accommodates terminal nitride (FeIV≡N) and dinitrogen (FeI–N2–FeI) ligands. J. Am. Chem. Soc. 126, 6252–6254 (2004).
Scepaniak, J. J., Young, J. A., Bontchev, R. P. & Smith, J. M. Formation of ammonia from an iron nitrido complex. Angew. Chem. Int. Ed. 48, 3158–3160 (2009).
Scepaniak, J. J. et al. Synthesis, structure, and reactivity of an iron(V) nitride. Science 331, 1049–1052 (2011).
Pickett, C. J. & Talarmin, J. Electrosynthesis of ammonia. Nature 317, 652–653 (1985).
Yandulov, D. V. & Schrock, R. R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 301, 76–78 (2003).
Arashiba, K., Miyake, Y. & Nishibayashi, Y. A molybdenum complex bearing PNP-type pincer ligands leads to the catalytic reduction of dinitrogen into ammonia. Nature Chem. 3, 120–125 (2011).
Fryzuk, M. D., Love, J. B., Rettig, S. J. & Young, V. G. Transformation of coordinated dinitrogen by reaction with dihydrogen and primary silanes. Science 275, 1445–1447 (1997).
Pool, J. A., Lobkovsky, E. & Chirik, P. J. Hydrogenation and cleavage of dinitrogen to ammonia with a zirconium complex. Nature 427, 527–530 (2004).
Schlögl, R. Catalytic synthesis of ammonia ‘a never-ending story'? Angew. Chem. Int. Ed. 42, 2004–2008 (2003).
Ertl, G. Reactions at surfaces: from atoms to complexity (Nobel Lecture). Angew. Chem. Int. Ed. 47, 3524–3535 (2008).
Honkala, K. et al. Ammonia synthesis from first-principles calculations. Science 307, 555–558 (2005).
Laplaza, C. E. & Cummins, C. C. Dinitrogen cleavage by a three-coordinate molybdenum(III) complex. Science 268, 861–863 (1995).
Zanotti-Gerosa, A. et al. Stepwise reduction of dinitrogen to nitride assisted by niobium bonded to oxygen donor atoms: the potential of reduced forms of niobium calix[4]arene. J. Am. Chem. Soc. 120, 437–438 (1998).
Clentsmith, G. K. B., Bates, V. M. E., Hitchcock, P. B. & Cloke, F. G. N. Reductive cleavage of dinitrogen by a vanadium diamidoamine complex: the molecular structures of [V(Me3SiN{CH2CH2NSiMe3}2)(µ-N)]2 and K[V(Me3SiN{CH2CH2NSiMe3}2)(µ-N)]2 . J. Am. Chem. Soc. 121, 10444–10445 (1999).
Solari, E. et al. Photochemical activation of the N≡N bond in a dimolybdenum–dinitrogen complex: formation of a molybdenum nitride. Angew. Chem. Int. Ed. 40, 3907–3909 (2001).
Kawaguchi, H. & Matsuo, T. Dinitrogen-bond cleavage in a niobium complex supported by a tridentate aryloxide ligand. Angew. Chem. Int. Ed. 41, 2792–2794 (2002).
Korobkov, I., Gambarotta, S. & Yap, G. P. A. A highly reactive uranium complex supported by the calix[4]tetrapyrrole tetraanion affording dinitrogen cleavage, solvent deoxygenation, and polysilanol depolymerization. Angew. Chem. Int. Ed. 41, 3433–3436 (2002).
Nikiforov, G. B., Vidyaratne, I., Gambarotta, S. & Korobkov, I. Titanium-promoted dinitrogen cleavage, partial hydrogenation, and silylation. Angew. Chem. Int. Ed. 48, 7415–7419 (2009).
Shaver, M. P. & Fryzuk, M. D. Activation of molecular nitrogen: coordination, cleavage and functionalization of N2 mediated by metal complexes. Adv. Synth. Catal. 345, 1061–1076 (2003).
Kubas, G. J. Fundamentals of H2 binding and reactivity on transition metals underlying hydrogenase function and H2 production and storage. Chem. Rev. 107, 4152–4205 (2007).
Caulton, K. G. The influence of π-stabilized unsaturation and filled/filled repulsions in transition metal chemistry. New J. Chem. 18, 25–41 (1994).
Walstrom, A. et al. A facile approach to a d4 Ru≡N: moiety. J. Am. Chem. Soc. 127, 5330–5331 (2005).
Brown, S. D., Mehn, M. P. & Peters, J. C. Heterolytic H2 activation mediated by low-coordinate L3Fe-(µ-N)-FeL3 complexes to generate Fe(µ-NH)(µ-H)Fe species. J. Am. Chem. Soc. 127, 13146–13147 (2005).
Vogel, C., Heinemann, F. W., Sutter, J., Anthon, C. & Meyer, K. An iron nitride complex. Angew. Chem. Int. Ed. 47, 2681–2684 (2008).
Scepaniak, J. J. et al. Structural and spectroscopic characterization of an electrophilic iron nitrido complex. J. Am. Chem. Soc. 130, 10515–10517 (2008).
Schöffel, J., Rogachev, A. Y., DeBeer George, S. & Burger, P. Isolation and hydrogenation of a complex with a terminal iridium–nitrido bond. Angew. Chem. Int. Ed. 48, 4734–4738 (2009).
Käß, M., Friedrich, A., Drees, M. & Schneider, S. Ruthenium complexes with cooperative PNP ligands: bifunctional catalysts for the dehydrogenation of ammonia–borane. Angew. Chem. Int. Ed. 48, 905–907 (2009).
Friedrich, A., Drees, M., Schmedt auf der Günne, J. & Schneider, S. Highly stereoselective proton/hydride exchange: assistance of hydrogen bonding for the heterolytic splitting of H2 . J. Am. Chem. Soc. 131, 17552–17553 (2009).
Askevold, B., Khusniyarov, M. M., Herdtweck, E., Meyer, K. & Schneider, S. A square-planar ruthenium(II) complex with a low-spin configuration. Angew. Chem. Int. Ed. 49, 7566–7569 (2010).
Watson, L. A., Ozerov, O. V., Pink, M. & Caulton, K. G. Four-coordinate, planar Ru(II). A triplet state as a response to a 14-valence electron configuration. J. Am. Chem. Soc. 125, 8426–8427 (2003).
Pap, J. S., DeBeer George, S. & Berry, J. F. Delocalized metal–metal and metal–ligand multiple bonding in a linear Ru–Ru≡N unit: elongation of a traditionally short Ru≡N bond. Angew. Chem. Int. Ed. 47, 10102–10105 (2008).
Musch Long, A. K., Yu, R. P., Timmer, G. H. & Berry, J. F. Aryl C–H bond amination by an electrophilic diruthenium nitride. J. Am. Chem. Soc. 132, 12228–12230 (2010).
Nugent, W. A. & Mayer, J. M. Metal Ligand Multiple Bonds (Wiley, 1988).
Eikey, R. A. & Abu-Omar, M. M. Nitrido and imido transition metal complexes of Groups 6–8. Coord. Chem. Rev. 243, 83–124 (2003).
Walstrom, A., Fan, H., Pink, M. & Caulton, K. G. Unexpected selectivity in electrophilic attack on (PNP)RuN. Inorg. Chim. Acta 363, 633–636 (2010).
Silvia, J. S. & Cummins, C. C. Two electron reduction of a vanadium(V) nitride by CO to release cyanate and open a coordination site. J. Am. Chem. Soc. 131, 446–447 (2008).
Tran, B. L., Pink, M., Gao, X., Park, H. & Mindiola, D. J. Low-coordinate and neutral nitrido complexes of vanadium. J. Am. Chem. Soc. 132, 1458–1459 (2010).
Mindiola, D. J. Early transition-metal hydrazido complexes: masked metallanitrenes from N–N bond scission. Angew. Chem. Int. Ed. 47, 1557–1559 (2008).
Ashcraft, R. W., Raman, S. & Green, W. H. Ab initio aqueous thermochemistry: application to the oxidation of hydroxylamine in nitric acid solution J. Phys. Chem. B 111, 11968–11983 (2007).
Schwarz, H. On the spin-forbiddeness of gas-phase ion–molecule reactions: a fruitful intersection of experimental and computational studies. Int. J. Mass Spectrom. 237, 75–105 (2004).
Poli, R. & Harvey, J. N. Spin forbidden chemical reactions of transition metal compounds. New ideas and new computational challenges. Chem. Soc. Rev. 32, 1–8 (2003).
Schröder, D., Shaik, S. & Schwarz, H. Two-state reactivity as a new concept in organometallic chemistry. Acc. Chem. Res. 33, 139–145 (2000).
Buncel, E. & Menon, B. Carbanion mechanisms. 6. Metalation of arylmethanes by potassium hydride/18-crown-6 ether in tetrahydrofuran and the acidity of hydrogen. J. Am. Chem. Soc. 99, 4457–4461 (1977).
Clapham, S. E., Hadzovic, A. & Morris, R. H. Mechanisms of the H2-hydrogenation and transfer hydrogenation of polar bonds catalyzed by ruthenium hydride complexes. Coord. Chem. Rev. 248, 2201–2237 (2004).
Grützmacher, H. Cooperating ligands in catalysis. Angew. Chem. Int. Ed. 47, 1814–1818 (2008).
Hölscher, M., Prechtl, M. H. G. & Leitner, W. Can [M(H)2(H2)(PXP)] pincer complexes (M = Fe, Ru, Os; X = N, O, S) serve as catalyst lead structures for NH3 synthesis from N2 and H2? Chem. Eur. J. 13, 6636–6643 (2007).
Hölscher, M. & Leitner, W. Lewis acid assisted stabilization of side-on bonded N2 in [Ru(NCN)]–pincer complexes – computational catalyst design directed at NH3 synthesis from N2 and H2 . Chem. Eur. J. 16, 14266–14271 (2010).
Acknowledgements
This work was partially funded by the Emmy Noether programme of the Deutsche Forschungsgemeinschaft (SCHN950/2-1). B.A. thanks the international graduate school NanoCat and the Technische Universität München Graduate School for support. The work in Frankfurt was supported by the Beilstein Institute as part of the NanoBiC research cooperative (project eNet). Computer time and excellent support, in particular by G. Laubender, was provided by the Center for Scientific Computing Frankfurt.
Author information
Authors and Affiliations
Contributions
B.A. and J.T.N. performed the synthetic work and spectrosopic examination, S.T. and M.D. the quantum chemical study and E.H. the crystallographic characterization, respectively. S.S. and M.C.H. designed and supervised the experimental and computational studies, respectively. The paper was written by B.A., S.T., M.D., M.C.H. and S.S. and all the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1253 kb)
Supplementary information
Crystallographic data for compound 5 (CIF 35 kb)
Supplementary information
Crystallographic data for compound 6·2 (CIF 47 kb)
Rights and permissions
About this article
Cite this article
Askevold, B., Nieto, J., Tussupbayev, S. et al. Ammonia formation by metal–ligand cooperative hydrogenolysis of a nitrido ligand. Nature Chem 3, 532–537 (2011). https://doi.org/10.1038/nchem.1051
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1051
This article is cited by
-
Near ambient N2 fixation on solid electrodes versus enzymes and homogeneous catalysts
Nature Reviews Chemistry (2023)
-
Ammonia synthesis by photocatalytic hydrogenation of a N2-derived molybdenum nitride
Nature Synthesis (2022)
-
A thiolate-bridged FeIVFeIV μ-nitrido complex and its hydrogenation reactivity toward ammonia formation
Nature Chemistry (2022)
-
Terminal uranium(V)-nitride hydrogenations involving direct addition or Frustrated Lewis Pair mechanisms
Nature Communications (2020)
-
Functionalization of N 2 to NH 3 via direct N ≡ N bond cleavage using M(III)(NMe 2 ) 3 (M=W/Mo): A theoretical study
Journal of Chemical Sciences (2015)