Abstract
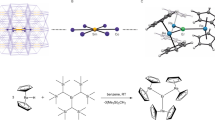
Single-molecule magnets (SMMs) are compounds that, below a blocking temperature, exhibit stable magnetization purely of molecular origin, and not caused by long-range ordering of magnetic moments in the bulk. They thus show promise for applications such as data storage of ultra-high density. The stability of the magnetization increases with increasing ground-state spin and magnetic anisotropy. Transition-metal SMMs typically possess high-spin ground states, but insufficient magnetic anisotropies. Lanthanide SMMs exhibit large magnetic anisotropies, but building high-spin ground states is difficult because they tend to form ionic bonds that limit magnetic exchange coupling. In contrast, the significant covalent bonding and large spin–orbit contributions associated with uranium are particularly attractive for the development of improved SMMs. Here we report a delocalized arene-bridged diuranium SMM. This study demonstrates that arene-bridged polyuranium clusters can exhibit SMM behaviour without relying on the superexchange coupling of spins. This approach may lead to increased blocking temperatures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sessoli, R., Gatteschi, D., Caneschi, A. & Novak, M. A. Magnetic bistability in a metal-ion cluster. Nature 365, 141–143 (1993).
Gatteschi, D., Sessoli, R. & Villain, J. Molecular Nanomagnets (Oxford Univ. Press, 2006).
Wernsdorfer, W., Aliaga-Alcalde, N., Hendrickson, D. N. & Christou, G. Exchange-biased quantum tunnelling in a supramolecular dimer of single-molecule magnets. Nature 416, 406–409 (2002).
Schelter, E. J., Prosvirin, A. V. & Dunbar, K. R. Molecular cube of Re-II and Mn-II that exhibits single-molecule magnetism. J. Am. Chem. Soc. 126, 15004–15005 (2004).
Manriquez, J. M., Yee, G. T., McLean, R. S., Epstein, A. J. & Miller, J. S. A room-temperature molecular/organic-based magnet. Science 252, 1415–1417 (1991).
Powell, A. K. et al. Synthesis, structures, and magnetic properties of Fe-2, Fe-17, and Fe-19 oxo-bridged iron clusters – the stabilization of high ground-state spins by cluster aggregates. J. Am. Chem. Soc. 117, 2491–2502 (1995).
Milios, C. J. et al. A record anisotropy barrier for a single-molecule magnet. J. Am. Chem. Soc. 129, 2754–2755 (2007).
Christou, G., Gatteschi, D., Hendrickson, D. N. & Sessoli, R. Single-molecule magnets. MRS Bull. 25, 66–71 (2000).
Leuenberger, M. N. & Loss, D. Quantum computing in molecular systems. Nature 410, 789–793 (2001).
Ardavan, A. et al. Will spin-relaxation times in molecular magnets permit quantum information processing? Phys. Rev. Lett. 98, 057201 (2007).
Winpenny, R. E. P. Quantum information processing using molecular nanomagnets as qubits. Angew. Chem. Int. Ed. 47, 7992–7994 (2008).
Manoli, M. et al. A ferromagnetic mixed-valent Mn supertetrahedron: towards low-temperature magnetic refrigeration with molecular clusters. Angew. Chem. Int. Ed. 46, 4456–4460 (2007).
Evangelisti, M. & Brechin, E. K. Recipes for enhanced molecular cooling. Dalton Trans. 39, 4672–4676 (2010).
Aromi, G. & Brechin, E. K. Synthesis of 3d metallic single-molecule magnets. Struct. Bonding 122, 1–67 (2006).
Rinehart, J. D., Harris, T. D., Kozimor, S. A., Bartlett, B. M. & Long, J. R. Magnetic exchange coupling in actinide-containing molecules. Inorg. Chem. 48, 3382–3395 (2009).
Sessoli, R. & Powell, A. K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 253, 2328–2341 (2009).
Lin, P.-H. et al. A polynuclear lanthanide single-molecule magnet with a record anisotropic barrier. Angew. Chem. Int. Ed. 48, 9489–9492 (2009).
Ishikawa, N., Sugita, M. & Wernsdorfer, W. Nuclear spin driven quantum tunneling of magnetization in a new lanthanide single-molecule magnet: bis(phthalocyaninato)holmium anion. J. Am. Chem. Soc. 127, 3650–3651 (2005).
Ishikawa, N., Sugita, M. & Wernsdorfer, W. Quantum tunneling of magnetization in lanthanide single-molecule magnets: bis(phthalocyaninato)terbium and bis(phthalocyaninato)dysprosium anions. Angew. Chem. Int. Ed. 44, 2931–2935 (2005).
Layfield, R. A. et al. Influence of the N-bridging ligand on magnetic relaxation in an organometallic dysprosium single-molecule magnet. Chem. Eur. J. 16, 4442–4446 (2010).
Newell, B. S., Rappé, A. K. & Shores, M. P. Experimental evidence for magnetic exchange in di- and trinuclear uranium(IV) ethynylbenzene complexes. Inorg. Chem. 49, 1595–1606 (2010).
Kozimor, S. A., Bartlett, B. M., Rinehart, J. D. & Long, J. R. Magnetic exchange coupling in chloride-bridged 5f–3d heterometallic complexes generated via insertion into a uranium(IV) dimethylpyrazolate dimer. J. Am. Chem. Soc. 129, 10672–10674 (2007).
Rosen, R. K., Andersen, R. A. & Edelstein, N. M. [(MeC5H4)3U]2[μ-1,4-N2C6H4]: a bimetallic molecule with antiferromagnetic coupling between the uranium centers. J. Am. Chem. Soc. 112, 4588–4590 (1990).
Salmon, L., Thuéry, P., Rivière E., Girerd, J.-J. & Ephritikhine, M. Structure and magnetism of the first strictly dinuclear compound containing paramagnetic 3d and 5f metal ions. Major influence of the CuII ion coordination on the exchange CuII–UIV interaction. Chem. Commun. 762–763 (2003).
Kiplinger, J. L. et al. Actinide-mediated cyclization of 1,2,4,5-tetracyanobenzene: synthesis and characterization of self-assembled trinuclear thorium and uranium macrocycles. Angew. Chem. Int. Ed. 45, 2036–2041 (2006).
Schelter, E. J. et al. Mixed valency in a uranium multimetallic complex. Angew. Chem. Int. Ed. 47, 2933–2996 (2008).
Spencer, L. P. et al. Cation–cation interactions, magnetic communication, and reactivity of the pentavalent uranium ion [U(NtBu)2]+. Angew. Chem. Int. Ed. 48, 3795–3798 (2009).
Arnold, P. L. et al. Synthesis of bimetallic uranium and neptunium complexes of a binucleating macrocycle and determination of the solid-state structure by magnetic analysis. Inorg. Chem. 49, 5341–5343 (2010).
Magnani, N. et al. Superexchange coupling and slow magnetic relaxation in a transuranium polymetallic complex. Phys. Rev. Lett. 104, 197202 (2010).
Nocton, G., Horeglad, P., Pécaut, J. & Mazzanti, M. Polynuclear cation–cation complexes of pentavalent uranyl: relating stability and magnetic properties to structure. J. Am. Chem. Soc. 130, 16633–16645 (2008).
Mougel, V., Horeglad, P., Nocton, G., Pécaut, J. & Mazzanti, M. Stable pentavalent uranyl species and selective assembly of a polymetallic mixed-valent uranyl complex by cation–cation interactions. Angew. Chem. Int. Ed. 48, 8477–8480 (2009).
Rinehart, J. D. & Long, J. R. Slow magnetic relaxation in a trigonal prismatic uranium(III) complex. J. Am. Chem. Soc. 131, 12558–12559 (2009).
Rinehart, J. D., Meihaus, K. R. & Long, J. R. Observation of secondary slow relaxation process for the field-induced single-molecule magnet U(H2BPz2)3 . J. Am. Chem. Soc. 132, 7572–7573 (2010).
Cooper, O. J., McMaster, J., Lewis, W., Blake, A. J. & Liddle, S. T. Synthesis and structure of [U{C(PPh2NMes)2}2] (Mes=2,4,6-Me3C6H2): a homoleptic uranium bis(carbene) complex with two formal U=C double bonds. Dalton Trans. 39, 5074–5076 (2010).
Ong, C. M. & Stephan, D. W. Lithiations of bis-diphenyl-N-trimethylsilylphosphiniminomethane: an X-ray structure of a 1,1-dilithiomethane derivative. J. Am. Chem. Soc. 121, 2939–2940 (1999).
Kasani, A., Kamalesh Babu, R. P., McDonald, R. & Cavell, R. G. [Ph2P(NSiMe3)]2CLi2: a dilithium dianionic methanide salt with an unusual Li4C2 cluster structure. Angew. Chem. Int. Ed. 38, 1483–1484 (1999).
Diaconescu, P. L., Arnold, P. L., Baker, T. A., Mindiola, D. J. & Cummins, C. C. Arene-bridged diuranium complexes: inverted sandwiches supported by δ backbonding. J. Am. Chem. Soc. 122, 6108–6109 (2000).
Evans, W. J., Kozimor, S. A., Ziller, J. W. & Kaltsoyannis, N. Structure, reactivity, and density functional theory analysis of the six-electron reductant, [(C5Me5)2U]2(μ-η6:η6-C6H6), synthesized via a new mode of (C5Me5)3M reactivity. J. Am. Chem. Soc. 126, 14533–14547 (2004).
Evans, W. J., Traina, C. A. & Ziller, J. W. Synthesis of heteroleptic uranium (μ-η6:η6-C6H6)2− sandwich complexes via facile displacement of (C5Me5)1− by ligands of lower hapticity and their conversion to heteroleptic bis(imido) compounds. J. Am. Chem. Soc. 131, 17473–17481 (2009).
Madelung, O. Landolt–Börnstein: Numerical Data and Functional Relationships in Science and Technology (Springer, 1987).
Cramer, R. E., Maynard, R. B., Paw, J. C. & Gilje, J. W. A uranium–carbon multiple bond. Crystal and molecular structure of (η5-C5H5)3UCHP(CH3)2(C6H5). J. Am. Chem. Soc. 103, 3589–3590 (1981).
Cantat, T. et al. The U=C double bond: synthesis and study of uranium nucleophilic carbene complexes. J. Am. Chem. Soc. 131, 963–672 (2009).
Tourneux, J.-C. et al. Easy access to uranium nucleophilic carbene complexes. Dalton Trans. 39, 2494–2496 (2010).
Avens, L. R. et al. A convenient entry into trivalent actinide chemistry: synthesis and characterization of AnI3(THF)4 and An[N(SiMe3)2]3 (An=U, Np, Pu). Inorg. Chem. 33, 2248–2256 (1994).
Carnall, W. T. A systematic analysis of the spectra of trivalent actinide chlorides in D3 h site symmetry. J. Chem. Phys. 96, 8713–8726 (1992).
Crosswhite, H. M., Crosswhite, H., Carnall, W. T. & Paszek, A. P. Spectrum analysis of U3+:LaCl3 . J. Chem. Phys. 72, 5103–5117 (1980).
Karbowiak, M. & Drożdżyński, J. Absorption spectrum analysis of uranium(III) formate. J. Alloys Compd 300–301, 329–333 (2000).
Carnall, W. T. & Wybourne, B. G. Electronic energy levels of the lighter actinides: U3+, Np3+, Pu3+, Am3+, and Cm3+. J. Chem. Phys. 40, 3428–3433 (1964).
Krupa, J. C. Optical excitations in lanthanide and actinide compounds. J. Alloys Compd 225, 1–10 (1995).
Dereń, P. J., Karbowiak, M., Krupa, J.-C. & Drożdżyński, J. Spectroscopic properties of U3+ ions in a ZnCl2-based glass. J. Alloys Compd 275–277, 393–397 (1998).
Graves, C. R. et al. Organometallic uranium(V)-imido halide complexes: from synthesis to electronic structure and bonding. J. Am. Chem. Soc. 130, 5272–5285 (2008).
Morris, D. E., Da Re, R. E., Jantunen, K. C., Castro-Rodriguez, I. & Kiplinger, J. L. Trends in electronic structure and redox energetics for early-actinide pentamethylcyclopentadienyl complexes. Organometallics 23, 5142–5153 (2004).
Liddle, S. T. et al. σ and π donation in an unsupported uranium–gallium bond. Angew. Chem. Int. Ed. 48, 1077–1080 (2009).
Jones, E. R., Hendricks, M. E., Stone, J. A. & Karraker, D. G. Magnetic properties of the trichlorides, tribromides, and triiodides of U(III), Np(III), and Pu(III). J. Chem. Phys. 60, 2088–2094 (1974).
Kahn, O. Molecular Magnetism (VCH, 1993).
Yang, E. C. et al. Fast magnetization tunnelling in tetranickel(II) single-molecule magnets. Inorg. Chem. 45, 529–546 (2006).
Petrukhina, M. A. Designed solvent-free approach toward organometallic networks built on directional metal–π-arene interactions. Coord. Chem. Rev. 251, 1690–1698 (2007).
Akita, M. & Koike, T. Chemistry of polycarbon species: from clusters to molecular devices. Dalton Trans. 3523–3530 (2008).
Acknowledgements
We thank the European Research Council, the UK Engineering and Physical Sciences Research Council, the University of Nottingham, the National Nuclear Laboratory and the Marie Curie Intra European Fellowship (F.M.) for support and funding, and the Royal Society for the award of a University Research Fellowship (S.T.L.).
Author information
Authors and Affiliations
Contributions
D.P.M. carried out the synthesis experiments and analysed the characterization data. F.M. and J.v.S. carried out and analysed the magnetic measurements data. J.M. carried out and analysed the DFT calculations. W.L. and A.J.B. carried out the X-ray single-crystal structure analyses. S.T.L. originated the central idea, supervised the work, analysed the data and wrote the manuscript, with contributions from all the co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1004 kb)
Supplementary information
Crystallographic data for compound 1 (CIF 27 kb)
Supplementary information
Crystallographic data for compound 2 (CIF 41 kb)
Supplementary information
Crystallographic data for compound 3 (CIF 28 kb)
Supplementary information
Crystallographic data for compound 4 (CIF 22 kb)
Supplementary information
Crystallographic data for compound 5 (CIF 33 kb)
Supplementary information
Crystallographic data for compound 6 (CIF 23 kb)
Supplementary information
Crystallographic data for compound 7 (CIF 25 kb)
Rights and permissions
About this article
Cite this article
Mills, D., Moro, F., McMaster, J. et al. A delocalized arene-bridged diuranium single-molecule magnet. Nature Chem 3, 454–460 (2011). https://doi.org/10.1038/nchem.1028
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1028
This article is cited by
-
Accessing five oxidation states of uranium in a retained ligand framework
Nature Communications (2023)
-
Photochemical Synthesis of Transition Metal-Stabilized Uranium(VI) Nitride Complexes
Nature Communications (2022)
-
Evidence for ligand- and solvent-induced disproportionation of uranium(IV)
Nature Communications (2021)
-
Electronic structure and magnetic properties of naphthalene- and stilbene-diimide-bridged diuranium(V) complexes: a theoretical study
Journal of Molecular Modeling (2020)
-
Transition-metal-bridged bimetallic clusters with multiple uranium–metal bonds
Nature Chemistry (2019)