Abstract
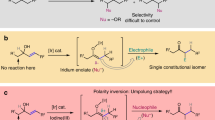
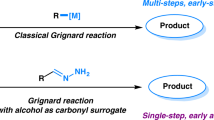
Methanol is an abundant (35 million metric tons per year), renewable chemical feedstock, yet its use as a one-carbon building block in fine chemical synthesis is highly underdeveloped. Using a homogeneous iridium catalyst developed in our laboratory, methanol engages in a direct C–C coupling with allenes to furnish higher alcohols that incorporate all-carbon quaternary centres, free of stoichiometric by-products. A catalytic mechanism that involves turnover-limiting methanol oxidation, a consequence of the high energetic demand of methanol dehydrogenation, is corroborated through a series of competition kinetics experiments. This process represents the first catalytic C–C coupling of methanol to provide discrete products of hydrohydroxymethylation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Anastas, P. & Eghbali, N. Green chemistry: principles and practice. Chem. Soc. Rev. 39, 301–312 (2010).
Sheldon, R. A. The E factor: fifteen years on. Green Chem. 9, 1273–1283 (2007).
Clark, J. H. Green chemistry for the second generation biorefinery – sustainable chemical manufacturing based on biomass. J. Chem. Tech. Biotech. 82, 603–609 (2007).
Lange, J.-P. Sustainable chemical manufacturing: a matter of resources, wastes, hazards and costs. ChemSusChem 2, 587–592 (2009).
The Methanol Institute: http://www.methanol.org/pdfFrame.cfm?pdf=WorldMeOHDemandbyDerivative.pdf (accessed 3 February 2011).
Chang, C. D. & Silvestri, A. J. Conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts. J. Catal. 47, 249–259 (1977).
Stöcker, M. Methanol-to-hydrocarbons: catalytic materials and their behavior. Micropor. Mesopor. Mater. 29, 3–48 (1999).
Olah, G. A. & Molnár, A. Hydrocarbon Chemistry 2nd edn, pp. 117–122 (Wiley, 2003).
Haw, J. F., Song, W., Marcus, D. M. & Nicholas, J. B. The mechanism of methanol to hydrocarbon catalysis. Acc. Chem. Res. 36, 317–326 (2003).
Bercaw, J. E. et al. Conversion of methanol to 2,2,3-trimethylbutane (triptane) over indium (III) iodide. Inorg. Chem. 46, 11371–11380 (2007).
Ahn, J. H., Temel, B. & Iglesia, E. Selective homologation routes to 2,2,3-trimethylbutane on solid acids. Angew. Chem. Int. Ed. 48, 3814–3816 (2009).
Kalck, P. & Serp, P. Iridium Complexes in Organic Synthesis (eds Oro, L. A. & Claver, C.) pp 195–209 (Wiley, 2008).
Haynes, A. Acetic acid synthesis by catalytic carbonylation of methanol. Top. Organomet. Chem. 18, 179–205 (2006).
Jones, J. H. The Cativa process for the manufacture of acetic acid. Platinum Met. Rev. 44, 94–105 (2000).
Maitlis, P. M., Haynes, A., Sunley, G. J. & Howard, M. J. Methanol carbonylation: thirty years on. J. Chem. Soc., Dalton Trans. 11, 2187–2196 (1996).
Jo, E.-A., Lee, J.-H. & Jun, C.-H. Rhodium(I)-catalyzed one-pot synthesis of dialkyl ketones from methanol and alkenes through directed sp3 C–H bond activation of N-methylamine. Chem. Commun. 44, 5779–5781 (2008).
Weissermel, K. & Arpe, H.-J. Industrial Organic Chemistry pp 127–144 (Wiley, 2003).
Patman, R. L., Bower, J. F., Kim, I. S. & Krische, M. J. Formation of C–C bonds via catalytic hydrogenation and transfer hydrogenation: vinylation, allylation, and enolate addition. Aldrichim. Acta 41, 95–104 (2008).
Bower, J. F., Kim, I. S., Patman, R. L. & Krische, M. J. Catalytic carbonyl addition through transfer hydrogenation: a departure from preformed organometallic reagents. Angew. Chem. Int. Ed. 48, 34–46 (2009).
Han, S. B., Kim, I. S. & Krische, M. J. Enantioselective iridium-catalyzed carbonyl allylation from the alcohol oxidation level via transfer hydrogenation: minimizing pre-activation for synthetic efficiency. Chem. Commun. 2009, 7278–7287 (2009).
Shibahara, F., Bower, J. F. & Krische, M. J. Ruthenium-catalyzed C–C bond forming transfer hydrogenation: carbonyl allylation from the alcohol or aldehyde oxidation level employing acyclic 1,3-dienes as surrogates to preformed allyl metal reagents. J. Am. Chem. Soc. 130, 6338–6339 (2008).
Shibahara, F., Bower, J. F. & Krische, M. J. Diene hydroacylation from the alcohol or aldehyde oxidation level via ruthenium-catalyzed C–C bond-forming transfer hydrogenation: synthesis of β,γ-unsaturated ketones. J. Am. Chem. Soc. 130, 14120–14122 (2008).
Han, H. & Krische, M. J. Direct ruthenium-catalyzed C–C coupling of ethanol: diene hydro-hydroxyethylation to form all-carbon quaternary centers. Org. Lett. 12, 2844–2846 (2010).
Patman, R. L., Chaulagain, M. R., Williams, V. M. & Krische, M. J. Direct vinylation of alcohols or aldehydes employing alkynes as vinyl donors: a ruthenium catalyzed C–C bond-forming transfer hydrogenation. J. Am. Chem. Soc. 131, 2066–2067 (2009).
Williams, V. M., Leung, J. C., Patman, R. L. & Krische, M. J. Hydroacylation of 2-butyne from the alcohol or aldehyde oxidation level via ruthenium catalyzed C–C bond forming transfer hydrogenation. Tetrahedron 65, 5024–5029 (2009).
Zbieg, J. R., McInturff, E. L. & Krische, M. J. Allenamide hydro-hydroxyalkylation: 1,2-amino alcohols via ruthenium-catalyzed carbonyl anti-aminoallylation. Org. Lett. 12, 2514–2516 (2010).
Bower, J. F., Patman, R. L. & Krische, M. J. Iridium-catalyzed C–C coupling via transfer hydrogenation: carbonyl addition from the alcohol or aldehyde oxidation level employing 1,3-cyclohexadiene. Org. Lett. 10, 1033–1035 (2008).
Zbieg, J. R., Fukuzumi, T. & Krische, M. J. Iridium catalyzed hydrohydroxyalkylation of butadiene: carbonyl crotylation. Adv. Synth. Catal. 352, 2416–2420 (2010).
Kim, I. S., Ngai, M.-Y. & Krische, M. J. Enantioselective iridium-catalyzed carbonyl allylation from the alcohol or aldehyde oxidation level using allyl acetate as an allyl metal surrogate. J. Am. Chem. Soc. 130, 6340–6341 (2008).
Kim, I. S., Ngai, M.-Y. & Krische, M. J. Enantioselective iridium-catalyzed carbonyl allylation from the alcohol or aldehyde oxidation level via transfer hydrogenative coupling of allyl acetate: departure from chirally modified allyl metal reagents in carbonyl addition. J. Am. Chem. Soc. 130, 14891–14899 (2008).
Kim, I. S., Han, S.-B. & Krische, M. J. anti-Diastereo- and enantioselective carbonyl crotylation from the alcohol or aldehyde oxidation level employing a cyclometallated iridium catalyst: α-methyl allyl acetate as a surrogate to preformed crotylmetal reagents. J. Am. Chem. Soc. 131, 2514–2520 (2009).
Lu, Y., Kim, I. S., Hassan, A., Del Valle, D. J. & Krische, M. J. 1,n-Glycols as dialdehyde equivalents in iridium-catalyzed enantioselective carbonyl allylation and iterative two-directional assembly of 1,3-polyols. Angew. Chem. Int. Ed. 48, 5018–5021 (2009).
Zhang, Y. J., Yang, J. H., Kim, S. H. & Krische, M. J. anti-Diastereo- and enantioselective carbonyl (hydroxymethyl) allylation from the alcohol or aldehyde oxidation level: allyl carbonates as allylmetal surrogates. J. Am. Chem. Soc. 132, 4562–4563 (2010).
Han, S. B., Kim, I.-S., Han, H. & Krische, M. J. Enantioselective carbonyl reverse prenylation from the alcohol or aldehyde oxidation level employing 1,1-dimethylallene as the prenyl donor. J. Am. Chem. Soc. 131, 6916–6917 (2009).
Qian, M., Liauw, M. A. & Emig, G. Formaldehyde synthesis from methanol over silver catalysts. Appl. Catal. A: Gen. 238, 211–222 (2003).
Lin, W.-H. & Chang, H.-F. A study of ethanol dehydration reaction in a palladium membrane reactor. Catal. Today 97, 181–188 (2004).
Yamagata, T., Iseki, A. & Tani, K. Preparation, properties and structures of [IrCl(diphosphine)]2 (diphosphine=(R)-BINAP and BPBP). Chem. Lett. 26, 1215–1216 (1997).
Tani, K., Iseki, A. & Yamagata, T. Efficient transfer hydrogenation of alkynes and alkenes with methanol catalysed by hydrido(methoxy)iridium(III) complexes. Chem. Commun. 1821–1822 (1999).
Smejkal, T., Han, H., Breit, B. & Krische, M. J. All-carbon quaternary centers via ruthenium-catalyzed hydroxymethylation of 2-substituted butadienes mediated by formaldehyde: beyond hydroformylation. J. Am. Chem. Soc. 131, 10366–10367 (2009).
Beaupére, D., Bauer, P., Nadjo, L. & Uzan, R. Transfer hydrogenation between alcohols and α,β-unsaturated ketones with RhH(PPh3)4 as catalyst. Evidence for regiospecificity and an unusual rate-limiting step. J. Organomet. Chem. 238, C12–C14 (1982).
Beaupére, D., Nadjo, L. & Uzan, R. New aspects of the transfer hydrogenation of α,β-unsaturated ketones by alcohols in the presence of hydridotetrakis(triphenylphosphine)rhodium(I) Part II. 1H NMR study and isotope effects. J. Mol. Catal. 20, 185–193 (1983).
Casey, C. P. & Johnson, J. B. Kinetic isotope effect evidence for a concerted hydrogen transfer mechanism in transfer hydrogenations catalyzed by [p-(Me2CH)C6H4Me]Ru(NHCHPhCHPhNSO2C6H4-p-CH3). J. Org. Chem. 68, 1998–2001 (2003).
Johnson, J. B. & Bäckvall, J.-E. Mechanism of ruthenium-catalyzed hydrogen transfer reactions: concerted transfer of OH and CH hydrogens from an alcohol to a (cyclopentadienone)ruthenium complex. J. Org. Chem. 68, 7681–7684 (2003).
Sandoval, C. A., Ohkuma, T., Muniz, K. & Noyori, R. Mechanism of asymmetric hydrogenation of ketones catalyzed by BINAP/1,2-diamine-ruthenium(II) complexes. J. Am. Chem. Soc. 125, 13490–13503 (2003).
Pannetier, N., Sortais, J.-B., Dieng, P. S., Barloy, L. Sirlin, C. & Pfeffer, M. Kinetics and mechanism of ruthenacycle-catalyzed asymmetric transfer hydrogenation. Organometallics 27, 5852–5859 (2008).
Stoutland, P. O., Bergman, R. G., Nolan, S. P. & Hoff, C. D. The thermodynamic driving force for C–H activation at iridium. Polyhedron 7, 1429–1440 (1988).
Acknowledgements
Acknowledgement is made to the Robert A. Welch Foundation (F-0038), the Texas Higher Education Coordinating Board (NHARP Award 01849), the University of Texas Center for Green Chemistry and Catalysis and the National Science Foundation (CHE-1021640). The Natural Sciences and Engineering Research Council of Canada and the German Research Foundation are thanked for partial support of J.M. and A.P., respectively.
Author information
Authors and Affiliations
Contributions
J.M., A.P. and R.A.M. carried out the experiments. M.J.K. directed the study. M.J.K. and J.M. co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4002 kb)
Supplementary information
Crystallographic data for compound DPPF-I (CIF 69 kb)
Rights and permissions
About this article
Cite this article
Moran, J., Preetz, A., Mesch, R. et al. Iridium-catalysed direct C–C coupling of methanol and allenes. Nature Chem 3, 287–290 (2011). https://doi.org/10.1038/nchem.1001
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1001
This article is cited by
-
Alternative sources of syngas for hydroformylation of unsaturated compounds
Russian Chemical Bulletin (2020)
-
N-formylation of amines using methanol as a potential formyl carrier by a reusable chromium catalyst
Communications Chemistry (2019)
-
The reductive C3 functionalization of pyridinium and quinolinium salts through iridium-catalysed interrupted transfer hydrogenation
Nature Chemistry (2019)
-
Visible light-driven C−H activation and C–C coupling of methanol into ethylene glycol
Nature Communications (2018)
-
C-Alkylation by Hydrogen Autotransfer Reactions
Topics in Current Chemistry (2016)