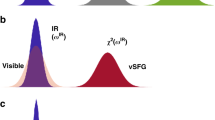
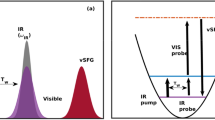
Abstract
Water is very different from liquids of similar molecular weight, and one of its unique properties is the very efficient transfer of vibrational energy between molecules, which arises as a result of strong dipole–dipole interactions between the O–H oscillators. Although we have a sound understanding of such energy transfer in bulk water, we know less about how, and how quickly, transfer occurs at its interface with a hydrophobic phase, because specifically addressing the outermost monolayer is difficult. Here, we use ultrafast two-dimensional surface-specific vibrational spectroscopy to probe the interfacial energy dynamics of heavy water (D2O) at the water/air interface. The measurements reveal the presence of surprisingly rapid energy transfer, both between hydrogen-bonded interfacial water molecules (intermolecular), and between O–D groups sticking out from the water surface and those located on the same molecule and pointing towards the water bulk (intramolecular). Vibrational energy transfer occurs on sub-picosecond timescales, and its rates and pathways can be quantified directly.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Du, Q., Superfine, R., Freysz, E. & Shen, Y. R. Vibrational spectroscopy of water at the vapor water interface. Phys. Rev. Lett. 70, 2313–2316 (1993).
Du, Q., Freysz, E. & Shen, Y. R. Surface vibrational spectroscopic studies of hydrogen-bonding and hydrophobicity. Science 264, 826–828 (1994).
Woutersen, S. & Bakker, H. J. Resonant intermolecular transfer of vibrational energy in liquid water. Nature 402, 507–509 (1999).
Lock, A. J., Woutersen, S. & Bakker, H. J. Ultrafast energy equilibration in hydrogen-bonded liquids. J. Phys. Chem. A 105, 1238–1243 (2001).
Cowan, M. et al. Ultrafast memory loss and energy redistribution in the hydrogen bond network of liquid H2O. Nature 434, 199–202 (2005).
Scatena, L., Brown, M. & Richmond, G. Water at hydrophobic surfaces: weak hydrogen bonding and strong orientation effects. Science 292, 908–912 (2001).
Shen, Y. R. & Ostroverkhov, V. Sum-frequency vibrational spectroscopy on water interfaces: polar orientation of water molecules at interfaces. Chem. Rev. 106, 1140–1154 (2006).
Gopalakrishnan, S., Liu, D. F., Allen, H. C., Kuo, M. & Shultz, M. J. Vibrational spectroscopic studies of aqueous interfaces: salts, acids, bases, and nanodrops. Chem. Rev. 106, 1155–1175 (2006).
Sovago, M., Campen, R. K., Bakker, H. J. & Bonn, M. Hydrogen bonding strength of interfacial water determined with surface sum-frequency generation. Chem. Phys. Lett. 470, 7–12 (2009).
Tian, C. S. & Shen, Y. R. Sum-frequency vibrational spectroscopic studies of water/vapor interfaces. Chem. Phys. Lett. 470, 1–6 (2009).
Nihonyanagi, S., Yamaguchi, S. & Tahara, T. Water hydrogen bond structure near highly charged interfaces is not like ice. J. Am. Chem. Soc. 132, 6867–6869 (2010).
Stiopkin, I. V. et al. Hydrogen bonding at the water surface revealed by isotopic dilution spectroscopy. Nature 474, 192–195 (2011).
Shen, Y. R. Surface-properties probed by 2nd harmonic and sum-frequency generation. Nature 337, 519–525 (1989).
Cho, M. H. Coherent two-dimensional optical spectroscopy. Chem. Rev. 108, 1331–1418 (2008).
Khalil, M., Demirdoven, N. & Tokmakoff, A. Coherent 2D IR spectroscopy: molecular structure and dynamics in solution. J. Phys. Chem. A 107, 5258–5279 (2003).
McGuire, J. A. & Shen, Y. R. Ultrafast vibrational dynamics at water interfaces. Science 313, 1945–1948 (2006).
Smits, M., Ghosh, A., Sterrer, M., Müller, M. & Bonn, M. Ultrafast vibrational energy transfer between surface and bulk water at the air–water interface. Phys. Rev. Lett. 98, 098302 (2007).
Bredenbeck, J., Ghosh, A., Nienhuys, H. K. & Bonn, M. Interface-specific ultrafast two-dimensional vibrational spectroscopy. Acc. Chem. Res. 42, 1332–1342 (2009).
Piatkowski, L., Eisenthal, K. B. & Bakker, H. J. Ultrafast intermolecular energy transfer in heavy water. Phys. Chem. Chem. Phys. 11, 9033–9038 (2009).
Lock, A. J. & Bakker, H. J. Temperature dependence of vibrational relaxation in liquid H2O. J. Chem. Phys. 117, 1708–1713 (2002).
Kraemer, D. et al. Temperature dependence of the two-dimensional infrared spectrum of liquid H2O. Proc. Natl Acad. Sci. USA 105, 437–442 (2008).
Kwak, K., Park, S., Finkelstein, I. J. & Fayer, M. D. Frequency-frequency correlation functions and apodization in two-dimensional infrared vibrational echo spectroscopy: a new approach. J. Chem. Phys. 127, 124503 (2007).
Asbury, J. B. et al. Dynamics of water probed with vibrational echo correlation spectroscopy. J. Chem. Phys. 121, 12431–12446 (2004).
Loparo, J. J., Roberts, S. T. & Tokmakoff, A. Multidimensional infrared spectroscopy of water. I. Vibrational dynamics in two-dimensional IR line shapes. J. Chem. Phys. 125, 194521 (2006).
Taylor, R. S., Dang, L. X. & Garrett, B. C. Molecular dynamics simulations of the liquid/vapor interface of SPC/E water. J. Phys. Chem. 100, 11720–11725 (1996).
Mucha, M. et al. Unified molecular picture of the surfaces of aqueous acid, base, and salt solutions. J. Phys. Chem. B 109, 7617–7623 (2005).
Gan, W., Wu, D., Zhang, Z., Feng, R. & Wang, H. Polarization and experimental configuration analyses of sum frequency generation vibrational spectra, structure, and orientational motion of the air/water interface. J. Chem. Phys. 124, 114705 (2006).
Bredenbeck, J., Ghosh, A., Smits, M. & Bonn, M. Ultrafast two dimensional-infrared spectroscopy of a molecular monolayer. J. Am. Chem. Soc. 130, 2152–2153 (2008).
Cervetto, V., Helbing, J., Bredenbeck, J. & Hamm, P. Double-resonance versus pulsed Fourier transform two-dimensional infrared spectroscopy: an experimental and theoretical comparison. J. Chem. Phys. 121, 5935–5942 (2004).
Acknowledgements
The authors thank J. Versluis and M. Jan van Zadel for their expert help and support and I. Cerjak for providing graphics.
Author information
Authors and Affiliations
Contributions
M.B. and H.J.B. designed the research project. Z.Z. and L.P. performed the experiments. M.B., Z.Z. and L.P. analysed the data. M.B. wrote the manuscript. All authors discussed the results, designed experiments and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 633 kb)
Rights and permissions
About this article
Cite this article
Zhang, Z., Piatkowski, L., Bakker, H. et al. Ultrafast vibrational energy transfer at the water/air interface revealed by two-dimensional surface vibrational spectroscopy. Nature Chem 3, 888–893 (2011). https://doi.org/10.1038/nchem.1158
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1158
This article is cited by
-
Hydrogen bond dynamics of interfacial water molecules revealed from two-dimensional vibrational sum-frequency generation spectroscopy
Scientific Reports (2021)
-
Reorientation-induced relaxation of free OH at the air/water interface revealed by ultrafast heterodyne-detected nonlinear spectroscopy
Nature Communications (2020)
-
Enhancement of the local asymmetry in the hydrogen bond network of liquid water by an ultrafast electric field pulse
Scientific Reports (2019)
-
Low-energy electrons transform the nimorazole molecule into a radiosensitiser
Nature Communications (2019)
-
Semiconductor quantum dots reveal dipolar coupling from exciton to ligand vibration
Communications Chemistry (2018)