Abstract
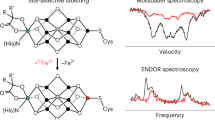
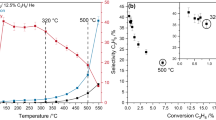
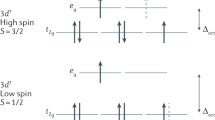
Oxo-transfer chemistry mediated by iron underpins many biological processes and today is emerging as synthetically very important for the catalytic oxidation of C–H and C=C moieties that are hard to activate conventionally. Despite the vast amount of research in this area, experimental characterization of the reactive species under catalytic conditions is very limited, although a Fe(V)=O moiety was postulated. Here we show, using variable-temperature mass spectrometry, the generation of a Fe(V)=O species within a synthetic non-haem complex at −40 °C and its reaction with an olefin. Also, with isotopic labelling we were able both to follow oxygen-atom transfer from H2O2/H2O through Fe(V)=O to the products and to probe the reactivity as a function of temperature. This study pioneers the implementation of variable-temperature mass spectrometry to investigate reactive intermediates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ortiz de Montellano, P. R. Cytochrome P450: Structure, Mechanism and Biochemistry 3rd edn (Kluwer Academic/Plenum, 2005).
Ferraro, D. J., Gakhar, L. & Ramaswamy, S. Rieske business: structure–function of Rieske non-heme oxygenases. Biochem. Biophys. Res. Commun. 338, 175–190 (2005).
Groves, J. T. High-valent iron in chemical and biological oxidations. J. Inorg. Biochem. 100, 434–447 (2006).
Chakrabarty, S., Austin, R. N., Deng, D., Groves, J. T. & Lipscomb, J. D. Radical intermediates in monooxygenase reactions of Rieske dioxygenases. J. Am. Chem. Soc. 129, 3514–3515 (2007).
Wolfe, M. D. et al. Benzoate 1,2-dioxygenase from Pseudomonas putida: single turnover kinetics and regulation of a two-component Rieske dioxygenase. Biochemistry 41, 9611–9626 (2002).
Schlichting, I. et al. The catalytic pathway of cytochrome P450cam at atomic resolution. Science 287, 1615–1622 (2000).
Rittle, J. & Green, M. T. Cytochrome P450 compound I: capture, characterization, and C–H bond activation kinetics. Science 330, 933–937 (2010).
Karlsson, A. et al. Crystal structure of naphthalene dioxygenase: side-on binding of dioxygen to iron. Science 299, 1039–1042 (2003).
Wolfe, M. D., Parales, J. V., Gibson, D. T. & Lipscomb, J. D. Single turnover chemistry and regulation of O2 activation by the oxygenase component of naphthalene 1,2-dioxygenase. J. Biol. Chem. 276, 1945–1953 (2001).
Chen, M. S. & White, M. C. A predictable selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science 318, 783–787 (2007).
Chen, M. S. & White, M. C. Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science 327, 566–571 (2010).
Gomez, L. et al. Stereospecific CH oxidation with H2O2 catalyzed by a chemically robust site-isolated iron catalyst. Angew. Chem. Int. Ed. 48, 5720–5723 (2009).
Que, L. & Tolman, W. B. Biologically inspired oxidation catalysis. Nature 455, 333–340 (2008).
Wolfe, M. D. & Lipscomb, J. D. Hydrogen peroxide-coupled cis-diol formation catalyzed by naphthalene 1,2-dioxygenase. J. Biol. Chem. 278, 829–835 (2003).
Chen, K. & Que, L. Jr Stereospecific alkane hydroxylation by nonheme iron catalysts: mechanistic evidence for an Fe(V)=O active species. J. Am. Chem. Soc. 123, 6327–6337 (2001).
Chen, K., Costas, M., Kim, J., Tipton, A. K. & Que, L. Jr Olefin cis-dihydroxylation versus epoxidation by nonheme iron catalysts: two faces of an Fe(III)–OOH coin. J. Am. Chem. Soc. 124, 3026–3035 (2002).
Bassan, A., Blomberg, M. R. A., Siegbahn, P. E. M. & Que, L. Jr Two faces of a biomimetic non-heme HO–Fe(V)=O oxidant: olefin epoxidation versus cis-dihydroxylation. Angew. Chem. Int. Ed. 44, 2939–2941 (2005).
Company, A. et al. Alkane hydroxylation by a nonheme iron catalyst that challenges the heme paradigm for oxygenase action. J. Am. Chem. Soc. 129, 15766–15767 (2007).
Company, A. et al. Olefin-dependent discrimination between two nonheme HO–Fe(V)=O tautomeric species in catalytic H2O2 epoxidations. Chem. Eur. J. 15, 3359–3362 (2009).
Makhlynets, O. V. & Rybak-Akimova, E. V. Aromatic hydroxylation at a non-heme iron center: observed intermediates and insights into the nature of the active species. Chem. Eur. J. 16, 13995–14006 (2010).
de Oliveira, F. T. et al. Chemical and spectroscopic evidence for an Fe(V)-oxo complex. Science 315, 835–838 (2007).
Markert, C. & Pfaltz, A. Screening of chiral catalysts and catalyst mixtures by mass spectrometric monitoring of catalytic intermediates. Angew. Chem. Int. Ed. 43, 2498–2500 (2004).
Feichtinger, D. & Plattner, D. A. Direct proof for O=Mn(V) (salen) complexes. Angew. Chem. Int. Ed. Engl. 36, 1718–1719 (1997).
Miras, H. N., Wilson, E. F. & Cronin, L. Unravelling the complexities of inorganic and supramolecular self-assembly in solution with electrospray and cryospray mass spectrometry. Chem. Commun. 1297–1311 (2009).
Wilson, E. F. et al. Probing the self-assembly of inorganic cluster architectures in solution with cryospray mass spectrometry: growth of polyoxomolybdate clusters and polymers mediated by silver(I) ions. J. Am. Chem. Soc. 130, 13876–13884 (2008).
Yan, J., Long, D.-L., Wilson, E. F. & Cronin, L. Discovery of heteroatom-embedded Te{W18O54} nanofunctional polyoxometalates by use of cryospray mass spectrometry. Angew. Chem. Int. Ed. 48, 4376–4380 (2009).
Lyakin, O. Y., Bryliakov, K. P., Britovsek, G. J. P. & Talsi, E. P. EPR spectroscopic trapping of the active species of nonheme iron-catalyzed oxidation. J. Am. Chem. Soc. 131, 10798–10799 (2009).
Ho, R. Y. N., Roelfes, G., Feringa, B. L. & Que, L. Jr Raman evidence for a weakened O–O bond in mononuclear low-spin iron(III)-hydroperoxides. J. Am. Chem. Soc. 121, 264–265 (1999).
Bassan, A., Blomberg, M. R. A., Siegbahn, P. E. M. & Que, L. Jr A density functional study of O–O bond cleavage for a biomimetic non-heme iron complex demonstrating an Fe(V)-intermediate. J. Am. Chem. Soc. 124, 11056–11063 (2002).
Quinonero, D., Morokuma, K., Musaev, D. G., Mas-Balleste, R. & Que, L. Jr Metal-peroxo versus metal-oxo oxidants in non-heme iron-catalyzed olefin oxidations: computational and experimental studies on the effect of water. J. Am. Chem. Soc. 127, 6548–6549 (2005).
Ensing, B., Buda, F. & Baerends, E. J. Fenton-like chemistry in water: oxidation catalysis by Fe(III) and H2O2 . J. Phys. Chem. A 107, 5722–5731 (2003).
Chow, T. W-S. et al. Cis-dihydroxylation of alkenes with Oxone catalyzed by iron complexes of a macrocyclic tetraaza ligand and reaction mechanism by ESI-MS spectrometry and DFT calculations. J. Am. Chem. Soc. 132, 13229–13239 (2010).
Comba, P., Rajaraman, G. & Rohwer, H. A. Density functional theory study of the reaction of the biomimetic iron(II) complex of a tetradentate bispidine ligand with H2O2 . Inorg. Chem. 46, 3826–3838 (2007).
Bukowski, M. R. et al. Catalytic epoxidation and 1,2-dihydroxylation of olefins with bispidine-iron(II)/H2O2 systems. Angew. Chem. Int. Ed. 45, 3446–3449 (2006).
Oldenburg, P. D., Feng, Y., Pryjomska-Ray, I., Ness, D. & Que, L. Jr Olefin cis-dihydroxylation with bio-inspired iron catalysts. Evidence for an Fe(II)/Fe(IV) catalytic cycle. J. Am. Chem. Soc. 132, 17713–17723 (2010).
Company, A. et al. Modelling the cis-oxo-labile binding site motif of non-heme iron oxygenases. Water exchange and remarkable oxidation reactivity of a novel non-heme iron(IV)-oxo compound bearing a tripodal tetradentate ligand. Chem. Eur. J. 17, 1622–1634 (2010).
Acknowledgements
We thank the Engineering and Physical Sciences Research Council and WestCHEM for funding, and Bruker Daltonics for collaboration. L.C. thanks the Royal Society and Wolfson Research for a merit award. M.C. and X.R. thank the Ministerio de Ciencia e Innovación (MICINN) for Project CTQ2009-08464, and Generalitat de Catalunya for Institució Catalana de Recerca i Estudis Avançats Academia Awards. M.C. thanks the European Research Foundation for Project ERC-2009-StG-239910. I.P. thanks MICINN for a PhD grant. L.C. and M.C. thank COST Action D40.
Author information
Authors and Affiliations
Contributions
L.C. and M.C. devised the initial concept for the work, L.C., M.C., X.R., J.S.M., I.P., J.M.L. and M.G. designed the experiments and J.S.M., M.G. and I.P. carried out the experiments and analysed the data. M.C. and L.C. co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1886 kb)
Rights and permissions
About this article
Cite this article
Prat, I., Mathieson, J., Güell, M. et al. Observation of Fe(V)=O using variable-temperature mass spectrometry and its enzyme-like C–H and C=C oxidation reactions. Nature Chem 3, 788–793 (2011). https://doi.org/10.1038/nchem.1132
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1132
This article is cited by
-
Characterized cis-FeV(O)(OH) intermediate mimics enzymatic oxidations in the gas phase
Nature Communications (2019)
-
Oxygen activation by mononuclear nonheme iron dioxygenases involved in the degradation of aromatics
JBIC Journal of Biological Inorganic Chemistry (2017)
-
Oxidation of alkane and alkene moieties with biologically inspired nonheme iron catalysts and hydrogen peroxide: from free radicals to stereoselective transformations
JBIC Journal of Biological Inorganic Chemistry (2017)
-
Dioxygen activation by nonheme iron enzymes with the 2-His-1-carboxylate facial triad that generate high-valent oxoiron oxidants
JBIC Journal of Biological Inorganic Chemistry (2017)
-
Facile Access to Graphene Oxide from Ferro-Induced Oxidation
Scientific Reports (2016)