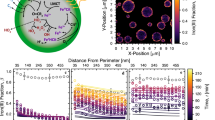
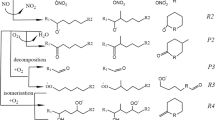
Abstract
Atmospheric aerosol particles play pivotal roles in climate and air quality. Just as chemically reduced gases experience oxidation in the atmosphere, it is now apparent that solid and liquid atmospheric particulates are also subject to similar oxidative processes. The most reactive atmospheric gas-phase radicals, in particular the hydroxyl radical, readily promote such chemistry through surficial interactions. This Review looks at progress made in this field, discussing the radical-initiated heterogeneous oxidation of organic and inorganic constituents of atmospheric aerosols. We focus on the kinetics and reaction mechanisms of such processes as well as how they can affect the physico–chemical properties of particles, such as their composition, size, density and hygroscopicity. Potential impacts on the atmosphere include the release of chemically reactive gases such as halogens, aldehydes and organic acids, reactive loss of particle-borne molecular tracer and toxic species, and enhanced hygroscopic properties of aerosols that may improve their ability to form cloud droplets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
IPCC in Climate Change 2007: The Physical Science Basis (eds Solomon, S. et al.) 1–18 (Cambridge Univ. Press, 2007).
Pope, C. A. III, Ezzati, M. & Dockery, D. W. Fine-particulate air pollution and life expectancy in the united states. N. Engl. J. Med. 360, 376–386 (2009).
Solomon, S. Stratospheric ozone depletion: A review of concepts and history. Rev. Geophys. 37, 275–316 (1999).
Robinson, A. L. et al. Rethinking organic aerosols: Semivolatile emissions and photochemical aging. Science 315, 1259–1262 (2007).
Kroll, J. H. & Seinfeld, J. H. Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere. Atmos. Environ. 42, 3593–3624 (2008).
Zhang, Q. et al. Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes. Geophys. Res. Lett. 34, L13801 (2007).
De Gouw, J. & Jimenez, J. L. Organic aerosols in the earth's atmosphere. Environ. Sci. Technol. 43, 7614–7618 (2009).
Rudich, Y. Laboratory perspectives on the chemical transformations of organic matter in atmospheric particles. Chem. Rev. 103, 5097–5124 (2003).
Rudich, Y., Donahue, N. M. & Mentel, T. F. Aging of organic aerosol: Bridging the gap between laboratory and field studies. Annu. Rev. Phys. Chem. 58, 321–352 (2007).
Atkinson, R. Gas-phase tropospheric chemistry of volatile organic compounds. 1. Alkanes and alkenes. J. Phys. Chem. 26, 215–290 (1997).
Cooper, P. L. & Abbatt, J. P. D. Heterogeneous interactions of OH and HO2 radicals with surfaces characteristic of atmospheric particulate matter. J. Phys. Chem. 100, 2249–2254 (1996).
Bertram, A. K., Ivanov, A. V., Hunter, M., Molina, L. T. & Molina, M. J. The reaction probability of OH on organic surfaces of tropospheric interest. J. Phys. Chem. A 105, 9415–9421 (2001).
Molina, M. J., Ivanov, A. V., Trakhtenberg, S. & Molina, L. T. Atmospheric evolution of organic aerosol. Geophys. Res. Lett. 31, L22104 (2004).
Moise, T. & Rudich, Y. Uptake of Cl and Br by organic surfaces - a perspective on organic aerosols processing by tropospheric oxidants. Geophys. Res. Lett. 28, 4083–4086 (2001).
Hearn, J. D. & Smith, G. D. A mixed-phase relative rates technique for measuring aerosol reaction kinetics. Geophys. Res. Lett. 33, L17805 (2006).
George, I. J., Vlasenko, A., Slowik, J. G., Broekhuizen, K. & Abbatt, J. P. D. Heterogeneous oxidation of saturated organic aerosols by hydroxyl radicals: uptake kinetics, condensed-phase products, and particle size change. Atmos. Chem. Phys. 7, 4187–4201 (2007).
Lambe, A. T., Zhang, J. Y., Sage, A. M. & Donahue, N. M. Controlled OH radical production via ozone-alkene reactions for use in aerosol aging studies. Environ. Sci. Technol. 41, 2357–2363 (2007).
Lambe, A. T., Miracolo, M. A., Hennigan, C. J., Robinson, A. L. & Donahue, N. M. Effective rate constants and uptake coefficients for the reactions of organic molecular markers (n-alkanes, hopanes, and steranes) in motor oil and diesel primary organic aerosols with hydroxyl radicals. Environ. Sci. Technol. 43, 8794–8800 (2009).
Hearn, J. D., Renbaum, L. H., Wang, X. & Smith, G. D. Kinetics and products from reaction of Cl radicals with dioctyl sebacate (DOS) particles in O2: a model for radical-initiated oxidation of organic aerosols. Phys. Chem. Chem. Phys. 9, 4803–4813 (2007).
Smith, J. D. et al. The heterogeneous reaction of hydroxyl radicals with sub-micron squalane particles: a model system for understanding the oxidative aging of ambient aerosols. Atmos. Chem. Phys. 9, 3209–3222 (2009).
McNeill, V. F., Yatavelli, R. L. N., Stipe, C. B. & Landgrebe, O. Heterogeneous OH oxidation of palmitic acid in single component and internally mixed aerosol particles: vaporization and role of particle phase. Atmos. Chem. Phys. 8, 5465–5476 (2008).
Moise, T., Talukdar, R. K., Frost, G. J., Fox, R. W. & Rudich, Y. Reactive uptake of NO3 by liquid and frozen organics. J. Geophys. Res. Atmos. 107, 4014 (2002).
Gross, S., Iannone, S. X., Xiao, S. & Bertram, A. K. Reactive uptake studies of NO3 and N2O5 on alkenoic acid, alkanoate, and polyalcohol substrates to probe nighttime aerosol chemistry. Phys. Chem. Chem. Phys. 11, 7792–7803 (2009).
Jech, D. D., Easley, P. G. & Krieger, B. B. Heterogeneous Atmospheric Chemistry 107–121 (American Geophysical Union, 1982).
Ivanov, A. V., Gershenzon, Y. M., Gratpanche, F., Devolder, P. & Sawerysyn, J.-P. Heterogeneous loss of OH on NaCl and NH4NO3 at tropospheric temperatures. Ann. Geophys. 14, 659–664 (1996).
Gershenzon, Y. M., Ivanov, A. V., Kucheryavyi, S. I. & Rozenshtein, V. B. Annihilation of OH radicals on the surfaces of substances chemically similar to atmospheric aerosol particles. Kinet. Catal. 27, 923–927 (1986).
Hanson, D. R., Burkholder, J. B., Howard, C. J. & Ravishankara, A. R. Measurement of OH and HO2 radical uptake coefficients on water and sulfuric-acid surfaces J. Phys. Chem. 96, 4979–4985 (1992).
Laskin, A. et al. A new approach to determining gas-particle reaction probabilities and application to the heterogeneous reaction of deliquesced sodium chloride particles with gas-phase hydroxyl radicals. J. Phys. Chem. A 110, 10619–27 (2006).
Davidovits, P., Kolb, C. E., Williams, L. R., Jayne, J. T. & Worsnop, D. R. Mass accommodation and chemical reactions at gas-liquid interfaces. Chem. Rev. 106, 1323–1354 (2006).
Renbaum, L. H. & Smtih, G. D. The importance of phase in the radical-initiated oxidation of model organic aerosols: reactions of solid and liquid brassidic acid particles. Phys. Chem. Chem. Phys. 11, 2441–2451 (2009).
Moise, T. & Rudich, Y. Reactive uptake of ozone by aerosol-associated unsaturated fatty acids: Kinetics, mechanism, and products. J. Phys. Chem. A 106, 6469–6476 (2002).
Hearn, J. D. & Smith, G. D. Measuring rates of reaction in supercooled organic particles with implications for atmospheric aerosol. Phys. Chem. Chem. Phys. 7, 2549–2551 (2005).
Katrib, Y. et al. Ozonolysis of mixed oleic-acid/stearic-acid particles: Reaction kinetics and chemical morphology. J. Phys. Chem. A 109, 10910–10919 (2005).
Knopf, D. A., Anthony, L. M. & Bertram, A. K. Reactive uptake of O3 by multicomponent and multiphase mixtures containing oleic acid. J. Phys. Chem. A 109, 5579–5589 (2005).
Hanson, D. R., Ravishankara, A. R. & Solomon, S. Heterogeneous reactions in sulfuric acid aerosols: A framework for model calculations. J. Geophys. Res. 99, 3615–3629 (1994).
Steinfeld, J. I., Francisco, J. S. & Hase W. L. Chemical Kinetics and Dynamics (Prentice Hall, 1998).
Bagot, P. A. J., Waring, C., Costen, M. L. & McKendrick, K. G. Dynamics of inelastic scattering of OH radicals from reactive and inert liquid surfaces. J. Phys. Chem. C 112, 10868–10877 (2008).
Vlasenko, A., George, I. J. & Abbatt, J. P. D. Formation of volatile organic compounds in the heterogeneous oxidation of condensed-phase organic films by gas-phase OH. J. Phys. Chem. A 112, 1552–1560 (2008).
Eliason, T. L., Gilman, J. B. & Vaida, V. Oxidation of organic films relevant to atmospheric aerosols. Atmos. Environ. 38, 1367–1378 (2004).
McNeill, V. F., Wolfe, G. M. & Thornton, J. A. The oxidation of oleate in submicron aqueous salt aerosols: Evidence of a surface process. J. Phys. Chem. A 111, 1073–1083 (2007).
Docherty, K. S. & Ziemann, P. J. Reaction of oleic acid particles with NO3 radicals: Products, mechanism, and implications for radical-initiated organic aerosol oxidation. J. Phys. Chem. A 110, 3567–3577 (2006).
Gross, S. & Bertram, A. K. Reactive uptake of NO3, N2O5, NO2, HNO3, and O3 on three types of polycyclic aromatic hydrocarbon surfaces. J. Phys. Chem. A 112, 3104–3113 (2008).
Russell, G. A. Deuterium-isotope effects in the autoxidation of aralkyl hydrocarbons — mechanism of the interaction of peroxy radicals. J. Am. Chem. Soc. 79, 3871–3877 (1957).
Knopf, D. A., Mak, J., Gross, S. & Bertram, A. K. Does atmospheric processing of saturated hydrocarbon surfaces by NO3 lead to volatilization? Geophys. Res. Lett. 33, L17816 (2006).
Renbaum, L. H. & Smith, G. D. Organic nitrate formation in the radical-initiated oxidation of model aerosol particles in the presence of NOx . Phys. Chem. Chem. Phys. 11, 8040–8047 (2009).
Gross, S. & Bertram, A. K. Products and kinetics of the reactions of an alkane monolayer and a terminal alkene monolayer with NO3 radicals. J. Geophys. Res. 114, D02307 (2009).
Kroll, J. H. et al. Measurement of fragmentation and functionalization pathways in the heterogeneous oxidation of oxidized organic aerosol. Phys. Chem. Chem. Phys. 11, 8005–8014 (2009).
Abbatt, J. P. D. Heterogeneous interactions of BrO and ClO: Evidence for BrO surface recombination and reaction with HSO3−/SO32−. Geophys. Res. Lett. 23, 1681–1684 (1996).
Loukhovitskaya, E., Bedjanian, Y., Morozov, I. & Le Bras, G. Laboratory study of the interaction of HO2 radicals with the NaCl, NaBr, MgCl2 and sea salt surfaces. Phys. Chem. Chem. Phys. 11, 7896–7905 (2009).
Oum, K. W., Lakin, M. J., DeHaan, D. O., Brauers, T. & Finlayson-Pitts, B. J. Formation of molecular chlorine from the photolysis of ozone and aqueous sea-salt particles. Science 279, 74–76 (1998).
Knipping, E. M. et al. Experiments and simulations of ion-enhanced interfacial chemistry on aqueous NaCl aerosols. Science 288, 301–306 (2000).
Finlayson-Pitts, B. J. The tropospheric chemistry of sea salt: A molecular-level view of the chemistry of NaCl and NaBr. Chem. Rev. 103, 4801–4822 (2003).
Roeselova, M., Jungwirth, P., Tobias, D. J. & Gerber, R. B. Impact, trapping, and accommodation of hydroxyl radical and ozone at aqueous salt aerosol surfaces. A molecular dynamics study. J. Phys. Chem. B 107, 12690–12699 (2003).
Frinak, E. K. & Abbatt, J. P. D. Br2 production from the heterogeneous reaction of gas-phase OH with aqueous salt solutions: impacts of acidity, halide concentration, and organic surfactants. J. Phys. Chem. A 110, 10456–10464 (2006).
Sjostedt, S. J. & Abbatt, J. P. D. Release of gas-phase halogens from sodium halide substrates: heterogeneous oxidation of frozen solutions and desiccated salts by hydroxyl radicals. Environ. Res. Lett. 3, 045007 (2008).
Zafiriou, O. C. Sources and reactions of OH and daughter radicals in seawater. J. Geophys. Res. 79, 4491–4497 (1974).
Rudich, Y., Talukdar, R. K., Ravishankara, A. R. & Fox, R. W. Reactive uptake of NO3 on pure water and ionic solutions. J. Geophys. Res. 101, 21023–21031 (1996).
Jiang, P.-Y. et al. Pulse radiolysis study of concentrated sulfuric acid solutions. Formation mechanism, yield and reactivity of sulfate radicals. J. Chem. Soc. Faraday Trans. 88, 1653–1658 (1992).
George, I. J., Slowik, J. & Abbatt, J. P. D. Chemical aging of ambient organic aerosol from heterogeneous reaction with hydroxyl radicals. Geophys. Res. Lett. 35, L13811 (2008).
Sage, A. M., Weitkamp, E. A., Robinson, A. L. & Donahue, N. M. Evolving mass spectra of the oxidized component of organic aerosol: results from aerosol mass spectrometer analyses of aged diesel emissions. Atmos. Chem. Phys. 8, 1139–1152 (2008).
Jimenez, J. L. et al. Evolution of organic aerosols in the atmosphere. Science 326, 1525–1529 (2009).
George, I. J. & Abbatt, J. P. D. Chemical evolution of secondary organic aerosol from OH-initiated heterogeneous oxidation. Atmos. Chem. Phys. 10, 5551–5563, (2010).
George, I. J. OH-initiated Heterogeneous Oxidation of Atmospheric Organic Aerosols PhD Thesis, Univ. Toronto (2009).
Aiken, A. C. et al. O/C and OM/OC ratios of primary, secondary, and ambient organic aerosols with high-resolution time-of-flight aerosol mass spectrometry. Environ. Sci. Tech. 42, 4478–4485 (2008).
Petters, M. D. et al. Chemical aging and the hydrophobic-to-hydrophilic conversion of carbonaceous aerosol. Geophys. Res. Lett. 33, L24806 (2006).
Kwan, A. J. et al. On the flux of oxygenated volatile organic compounds from organic aerosol oxidation. Geophys. Res. Lett. 33, L15815 (2006).
Lim, Y. B. & Ziemann, P. J. Effects of molecular structure on aerosol yields from OH radical-initiated reactions of linear, branched, and cyclic alkanes in the presence of NO. Environ. Sci. Technol. 43, 2328–2334 (2009).
George, I. J., Chang, R. Y.-W., Danov, V., Vlasenko, A. & Abbatt, J. P. D. Modification of cloud condensation nucleus activity of organic aerosols by hydroxyl radical heterogeneous oxidation. Atmos. Environ. 43, 5038–5045 (2009).
Robinson, A. L., Donahue, N. M. & Rogge W. F. Photochemical oxidation and changes in molecular composition of organic aerosol in the regional context. J. Geophys. Res. 111, D03302 (2006).
Kanakidou, M. et al. Organic aerosol and global climate modelling: a review. Atmos. Chem. Phys. 5, 1053–1123 (2005).
Prinn, R. G. et al. Evidence for substantial variations of atmospheric hydroxyl radicals in the past two decades. Science 292, 1882–1888 (2001).
Heald, C. L. et al. A simplified description of the evolution of organic aerosol composition in the atmosphere. Geophys. Res. Lett. 37, L08803 (2010).
Barrie, L. A., Bottenheim, J. W., Schnell, R. C., Crutzen, P. J. & Rasmussen, R. A Ozone destruction and photochemical reactions at polar sunrise in the lower Arctic atmosphere. Nature 334, 138–141 (1988).
Vogt, R., Crutzen, P. J. & Sander, R. A mechanism for halogen release from sea-salt aerosol in the remote marine boundary layer. Nature 383, 327–330 (1996).
Adams, J. W., Holmes, N. S. & Crowley, J. N. Uptake and reaction of HOBr on frozen and dry NaCl/NaBr surfaces between 253 and 233K. Atmos. Chem. Phys. 2, 79–91 (2002).
Matthew, B. M., George, I. & Anastasio, C. Hydroperoxyl radical (HO2) oxidizes dibromide radical anion (Br2−) to bromine (Br2) in aqueous solution: Implications for the formation of Br2 in the marine boundary layer. Geophys. Res. Lett. 30, 2297 (2003).
Laskin, A. et al. Reactions at interfaces as a source of sulfate formation in sea-salt particles. Science 301, 340–344 (2003).
Petters, M. D. & Kreidenweis, S. M. A single parameter representation of hygroscopic growth and cloud condensation nucleus activity. Atmos. Chem. Phys. 7, 1961–1971 (2007).
Acknowledgements
The work in this field done by the authors has been largely supported by NSERC (Canada).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
George, I., Abbatt, J. Heterogeneous oxidation of atmospheric aerosol particles by gas-phase radicals. Nature Chem 2, 713–722 (2010). https://doi.org/10.1038/nchem.806
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.806
This article is cited by
-
Atmospheric heterogeneous reaction of chlorobenzene on mineral α-Fe2O3 particulates: a chamber experiment study
Frontiers of Environmental Science & Engineering (2023)
-
In situ analysis of the bulk and surface chemical compositions of organic aerosol particles
Communications Chemistry (2022)
-
Chemical composition and radiative forcing of atmospheric aerosols over the high-altitude Western Himalayas of India
Environmental Science and Pollution Research (2022)
-
Heterogeneous Reaction of OH Radicals with Terbuthylazine on Self-synthesized Silica Particles in an Aerosol Smog Chamber at Different Temperatures
Aerosol Science and Engineering (2021)
-
Temperature dependence of rate constants for the H(D) + CH4 reaction in gas and aqueous phase: deformed Transition-State Theory study including quantum tunneling and diffusion effects
Structural Chemistry (2020)