Abstract
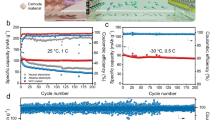
Aqueous lithium-ion batteries may solve the safety problem associated with lithium-ion batteries that use highly toxic and flammable organic solvents, and the poor cycling life associated with commercialized aqueous rechargeable batteries such as lead-acid and nickel-metal hydride systems. But all reported aqueous lithium-ion battery systems have shown poor stability: the capacity retention is typically less than 50% after 100 cycles. Here, the stability of electrode materials in an aqueous electrolyte was extensively analysed. The negative electrodes of aqueous lithium-ion batteries in a discharged state can react with water and oxygen, resulting in capacity fading upon cycling. By eliminating oxygen, adjusting the pH values of the electrolyte and using carbon-coated electrode materials, LiTi2(PO4)3/Li2SO4/LiFePO4 aqueous lithium-ion batteries exhibited excellent stability with capacity retention over 90% after 1,000 cycles when being fully charged/discharged in 10 minutes and 85% after 50 cycles even at a very low current rate of 8 hours for a full charge/discharge offering an energy storage system with high safety, low cost, long cycling life and appropriate energy density.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Li, W., Dahn, J. R. & Wainwright, D. Rechargeable lithium batteries with aqueous-electrolytes. Science 264, 1115–1118 (1994).
Kohler, J., Makihara, H., Uegaito, H., Inoue, H. & Toki, M. LiV3O8: characterization as anode materials for an aqueous rechargeable Li-ion battery system. Electrochim. Acta. 46, 59–66 (2000).
Wang, G. J., Fu, L. J., Zhao, N. H., Yang, L. C., Wu, Y. P. & Wu, H. Q. An aqueous rechargeable lithium battery with good cycling performance. Angew. Chem. Int. Ed. 46, 295–297 (2007).
Wang, H. B., Huang, K. L., Zeng, Y. Q., Yang, S. & Chen, L. Q. Electrochemical properties of TiP2O7 and LiTi2(PO4)3 as anode materials for lithium ion battery with aqueous solution electrolyte. Electrochim. Acta. 52, 3280–3286 (2007).
Luo, J. Y. & Xia, Y. Y. Aqueous lithium-ion battery LiTi2(PO4)3/LiMn2O4 with high power and energy densities as well as superior cycling stability. Adv. Funct. Mater. 17, 3877–3884 (2007).
Huang, H., Yin, S. C. & Nazar, L. F. Approaching theoretical capacity of LiFePO4 at room temperature at high rates. Electrochem. Solid State Lett. 4, A170–A172 (2001).
Li, W., McKinnon, W. R. & Dahn, J. R. Lithium intercalation from aqueous-solution. J. Electrochem. Soc. 141, 2310–2316 (1994).
Li, W. & Dahn, J. R. Lithium-ion cells with aqueous electrolytes. J. Electrochem. Soc. 142, 1472–1746 (1995).
Zhang, M. J. & Dahn, J. R. Electrochemical lithium intercalation in VO2(B) in aqueous electrolyte. J. Electrochem. Soc. 143, 2730–2734 (1996).
Dahn, J. R., Von Sacken, U., Juzkow, M. W. & Al Janaby, H. Rechargeable LiNiO2 carbon cells. J. Electrochem. Soc. 138, 2207–2211 (1991).
McKinnon, W. R. & Haering, R. R. in Mordern Aspects of Electrochemistry (eds White, R. E., Bockris, J. O'M. & Conway, B. E.) No. 15 (Plenum Press, 1983).
Choi, J., Alvarez, E., Arunkumar, T. & Manthiram, A. Proton insertion into oxide cathode during chemical delithiation. Electrochem. Solid State Lett. 9, A241–A244 (2006).
Choi, J. & Manthiram, A. Chemical and structural instabilities of lithium ion battery cathode. J. Power Sources 159, 249–253 (2006).
Wang, Y. G., Luo, J. Y., Wang, C. X. & Xia, Y. Y. Hybrid aqueous energy storage cells using activated carbon and lithium-ion intercalated compound II. Comparison of LiMn2O4, LiCo1/3Ni1/3Mn1/3O2, and LiCoO2 positive electrodes. J. Electrochem. Soc. 153, A1425–A1431 (2006).
Yu, D. Y. W. et al. Impurities in LiFePO4 and their influence on material characteristics. J. Electrochem. Soc. 155, A526–A530 (2008).
Acknowledgements
We acknowledge the support of the National Natural Science Foundation of China (20633040, 20925312), the State Key Basic Research Program of PRC (2007CB209703), and Shanghai Science & Technology Committee (09XD1400300, 08DZ2270500).
Author information
Authors and Affiliations
Contributions
J.L., W.C., P.H. and Y.X. conceived and designed the experiments, analysed and discussed results and commented on the manuscript. J.L. and W.C. performed the experiments and analysed the data. J.L. and Y.X. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 748 kb)
Rights and permissions
About this article
Cite this article
Luo, JY., Cui, WJ., He, P. et al. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nature Chem 2, 760–765 (2010). https://doi.org/10.1038/nchem.763
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.763
This article is cited by
-
Effects of conductive agent type on lithium extraction from salt lake brine with LiFePO4 electrodes
International Journal of Minerals, Metallurgy and Materials (2024)
-
Impact of KNO3 Concentration on Structural Properties, Dielectric and AC Conductivity Response of 80 PEO:20 PVDF Blend Polymer Electrolytes
Korean Journal of Chemical Engineering (2024)
-
B-VO2 nanowires: controlled hydrothermal synthesis and optical properties
Journal of the Australian Ceramic Society (2024)
-
Recent progress on electro-sorption technology for lithium recovery from aqueous sources
Nano Research (2024)
-
Water-in-salt electrolytes made saltier by Gemini ionic liquids for highly efficient Li-ion batteries
Scientific Reports (2023)