Abstract
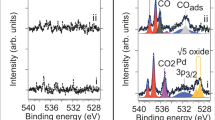
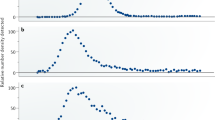
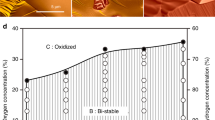
Atomic steps at the surface of a catalyst play an important role in heterogeneous catalysis, for example as special sites with increased catalytic activity. Exposure to reactants can cause entirely new structures to form at the catalyst surface, and these may dramatically influence the reaction by ‘poisoning’ it or by acting as the catalytically active phase. For example, thin metal oxide films have been identified as highly active structures that form spontaneously on metal surfaces during the catalytic oxidation of carbon monoxide. Here, we present operando X-ray diffraction experiments on a palladium surface during this reaction. They reveal that a high density of steps strongly alters the stability of the thin, catalytically active palladium oxide film. We show that stabilization of the metal, caused by the steps and consequent destabilization of the oxide, is at the heart of the well-known reaction rate oscillations exhibited during CO oxidation at atmospheric pressure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Taylor, H. S. A theory of the catalytic surface. Proc. R. Soc. Lond. A 108, 105–111 (1925).
Yates, J. T. Surface chemistry at metallic defect sites. J. Vac. Sci. Technol. A 13, 1359–1367 (1995).
Hammer, B., Nielsen, O. H. & Nørskov, J. K. Structure sensitivity in adsorption: CO interaction with stepped and reconstructed Pt surfaces. Catal. Lett. 46, 31–35 (1997).
Somorjai, G. A. Introduction to Surface Chemistry and Catalysis (Wiley, 1994).
Zambelli, T., Wintterlin, J., Trost, J. & Ertl, G. Identification of the ‘active sites’ of a surface-catalyzed reaction. Science 273, 1688–1690 (1996).
Dahl, S. et al. Role of steps in N2 activation on Ru(0001). Phys. Rev. Lett. 83, 1814–1817 (1999).
Geerlings, J. J. C. et al. Fischer–Tropsch technology—from active site to commercial process. Appl. Catal. A 186, 27–40 (1999).
Vang, R. T. et al. Controlling the catalytic bond-breaking selectivity of Ni surfaces by step blocking. Nature Mater. 4, 160–162 (2005).
Over, H. et al. Atomic scale structure and catalytic reactivity of the RuO2(110) surface. Science 287, 1474–1476 (2000).
Hendriksen, B. L. M. & Frenken, J. W. M. CO oxidation on Pt(110): scanning tunneling microscopy inside a flow reactor. Phys. Rev. Lett. 89, 046101 (2002).
Ackermann, M. D. et al. Structure and reactivity of surface oxides on Pt(110) during catalytic CO oxidation. Phys. Rev. Lett. 95, 255505 (2005).
Lundgren, E. et al. Kinetic hindrance during the initial oxidation of Pd(100) at ambient pressures. Phys. Rev. Lett. 92, 046101 (2004).
Reuter, K. & Scheffler, M. First-principles atomistic thermodynamics for oxidation catalysis: surface phase diagrams and catalytically interesting regions. Phys. Rev. Lett. 90, 046103 (2003).
Gao, F., Wang, Y., Cai, Y. & Goodman D. W. CO oxidation on Pt-group metals from ultrahigh vacuum to near atmospheric pressures. 2. Palladium and platinum. J. Phys. Chem. C 113, 174–181 (2009).
Rogal, J., Reuter, K. & Scheffler, M. CO oxidation at Pd(100): a first-principles constrained thermodynamics study. Phys. Rev. B 75, 205433 (2007).
Mars, P. & Van Krevelen, D. W. Oxidation carried out by means of vanadium oxide catalysts. Spec. Suppl. Chem. Eng. Sci. 3, 41–57 (1954).
Hendriksen, B. L. M., Bobaru, S. C. & Frenken, J. W. M. Oscillatory CO oxidation on Pd(100) studied with in situ scanning tunneling microscopy. Surf. Sci. 552, 229–242 (2004).
Hendriksen, B. L. M., Bobaru, S. C. & Frenken, J. W. M. Bistability and oscillations in CO oxidation studied with scanning tunneling microscopy inside a reactor. Catal. Today 105, 234–243 (2005).
Imbihl, R. & Ertl, G. Oscillatory kinetics in heterogeneous catalysis. Chem. Rev. 95, 697–733 (1995).
Schüth, F., Henry, B. E. & Schmidt, L. D. Oscillatory reactions in heterogeneous catalysis. Adv. Catal. 39, 51–127 (1993).
Robinson, I. K. Crystal truncation rods and surface roughness. Phys. Rev. B 33, 3830–3836 (1986).
Stierle, A. et al. A surface X-ray study of the structure and morphology of the oxidized Pd(001) surface. J. Chem. Phys. 122, 044706 (2005).
Wagner, C. The formation of thin oxide films on metals. Corrosion Sci. 13, 23–52 (1973).
Klikovits, J. et al. Step-orientation-dependent oxidation: from 1D to 2D oxides. Phys. Rev. Lett. 101, 266104 (2008).
Thostrup, P. et al. Adsorption-induced step formation. Phys. Rev. Lett. 87, 126102 (2001).
Zhang, Y., Rogal, J. & Reuter, K. Density-functional theory investigation of oxygen adsorption at Pd(11N) vicinal surfaces (N=3,5,7): influence of neighboring steps. Phys. Rev. B 74, 125414 (2006).
Williams, E. D. & Bartelt, N. C. Thermodynamics of surface morphology. Science 251, 393–400 (1991).
Sales, B. C., Turner, J. E. & Maple, M. B. Oscillatory oxidation of CO over Pt, Pd and Ir catalysts: theory. Surf. Sci. 114, 381–394 (1982).
Acknowledgements
The authors acknowledge E. Lundgren for providing a palladium crystal and the ESRF staff for support. This work has been financially supported by the Stichting Technische Wetenschappen (STW), the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) and the European Commission under contract no. NMP3-CT-2003-505670 (NANO2).
Author information
Authors and Affiliations
Contributions
B.L.M.H. and J.W.M.F. conceived the new explanation for the oscillatory reaction and B.L.M.H. developed the corresponding numerical model. M.D.A. and I.P. performed the batch experiments. R.v.R., D.S., O.B., A.R. and D.W. performed the flow experiments. M.D.A., R.v.R. and B.L.M.H. analysed the data. R.F. and S.F. supervised the experiments and J.W.M.F. supervised the project. B.L.M.H., M.D.A., R.v.R. and J.W.M.F. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1052 kb)
Supplementary information
Supplementary movie S1 (MOV 3259 kb)
Supplementary information
Supplementary movie S2 (MOV 2370 kb)
Rights and permissions
About this article
Cite this article
Hendriksen, B., Ackermann, M., van Rijn, R. et al. The role of steps in surface catalysis and reaction oscillations. Nature Chem 2, 730–734 (2010). https://doi.org/10.1038/nchem.728
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.728
This article is cited by
-
Metastable nickel–oxygen species modulate rate oscillations during dry reforming of methane
Nature Catalysis (2024)
-
Periodic structural changes in Pd nanoparticles during oscillatory CO oxidation reaction
Nature Communications (2022)
-
Stroboscopic operando spectroscopy of the dynamics in heterogeneous catalysis by event-averaging
Nature Communications (2021)
-
In situ observation of oscillatory redox dynamics of copper
Nature Communications (2020)
-
Surface-reaction induced structural oscillations in the subsurface
Nature Communications (2020)