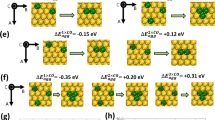
Abstract
The facile decomposition of ammonia to produce hydrogen is critical to its use as a hydrogen storage medium in a hydrogen economy, and although ruthenium shows good activity for catalysing this process, its expense and scarcity are prohibitive to large-scale commercialization. The need to develop alternative catalysts has been addressed here, using microkinetic modelling combined with density functional studies to identify suitable monolayer bimetallic (surface or subsurface) catalysts based on nitrogen binding energies. The Ni–Pt–Pt(111) surface, with one monolayer of Ni atoms residing on a Pt(111) substrate, was predicted to be a catalytically active surface. This was verified using temperature-programmed desorption and high-resolution electron energy loss spectroscopy experiments. The results reported here provide a framework for complex catalyst discovery. They also demonstrate the critical importance of combining theoretical and experimental approaches for identifying desirable monolayer bimetallic systems when the surface properties are not a linear function of the parent metals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ganley, J. C., Thomas, F. S., Seebauer, E. G. & Masel, R. I. A priori catalytic activity correlations: the difficult case of hydrogen production from ammonia. Catal. Lett. 96, 117–122 (2004).
Choudhary, T. V., Sivadinarayana, C. & Goodman, D. W. Catalytic ammonia decomposition: COx-free hydrogen production for fuel cell applications. Catal. Lett. 72, 197–201 (2001).
Yin, S. F. et al. Investigation on the catalysis of COx-free hydrogen generation from ammonia. J. Catal. 224, 384–396 (2004).
Jacobsen, C. J. H. et al. Catalyst design by interpolation in the periodic table: bimetallic ammonia synthesis catalysts. J. Am. Chem. Soc. 123, 8404–8405 (2001).
Boisen, A., Dahl, S., Norskov, J. K. & Christensen, C. H. Why the optimal ammonia synthesis catalyst is not the optimal ammonia decomposition catalyst. J. Catal. 230, 309–312 (2005).
Campbell, C. T. Bimetallic surface-chemistry. Annu. Rev. Phys. Chem. 41, 775–837 (1990).
Tao, F. et al. Reaction-driven restructuring of Rh–Pd and Pt–Pd core–shell nanoparticles. Science 322, 932–934 (2008).
Ruban, A. V., Skriver, H. L. & Norskov, J. K. Surface segregation energies in transition-metal alloys. Phys. Rev. B 59, 15990–16000 (1999).
Menning, C. A. & Chen, J. G. General trend for adsorbate-induced segregation of subsurface metal atoms in bimetallic surfaces. J. Chem. Phys. 130, 174709 (2009).
Kitchin, J. R., Reuter, K. & Scheffler, M. Alloy surface segregation in reactive environments: first-principles atomistic thermodynamics study of Ag3Pd(111) in oxygen atmospheres. Phys. Rev. B 77, 075437 (2008).
Ma, Y. G. & Balbuena, P. B. Pt surface segregation in bimetallic Pt3M alloys: a density functional theory study. Surf. Sci. 602, 107–113 (2008).
Chen, J. G., Menning, C. A. & Zellner, M. B. Monolayer bimetallic surfaces: experimental and theoretical studies of trends in electronic and chemical properties. Surf. Sci. Rep. 63, 201–254 (2008).
Pallassana, V., Neurock, M., Hansen, L. B., Hammer, B. & Norskov, J. K. Theoretical analysis of hydrogen chemisorption on Pd(111), Re(0001) and Pd-ML/Re(0001), Re-ML/Pd(111) pseudomorphic overlayers. Phys. Rev. B 60, 6146–6154 (1999).
Rodriguez, J. A. & Goodman, D. W. The nature of the metal metal bond in bimetallic surfaces. Science 257, 897–903 (1992).
Mhadeshwar, A. B., Kitchin, J. R., Barteau, M. A. & Vlachos, D. G. The role of adsorbate−adsorbate interactions in the rate controlling step and the most abundant reaction intermediate of NH3 decomposition on Ru. Catal. Lett. 96, 13–22 (2004).
Shustorovich, E. & Sellers, H. The UBI-QEP method: a practical theoretical approach to understanding chemistry on transition metal surfaces. Surf. Sci. Rep. 31, 5–119 (1998).
Campbell, C. T. Finding the rate-determining step in a mechanism—comparing DeDonder relations with the ‘degree of rate control’. J. Catal. 204, 520–524 (2001).
Campbell, C. T. Micro- and macro-kinetics: their relationship in heterogeneous catalysis. Top. Catal. 1, 353–366 (1994).
Menning, C. A., Hwu, H. H. & Chen, J. G. Experimental and theoretical investigation of the stability of Pt–3d–Pt(111) bimetallic surfaces under oxygen environment. J. Phys. Chem. B 110, 15471–15477 (2006).
Humbert, M. P. & Chen, J. G. Correlating hydrogenation activity with binding energies of hydrogen and cyclohexene on M/Pt(111) (M=Fe, Co, Ni, Cu) bimetallic surfaces. J. Catal. 257, 297–306 (2008).
Kitchin, J. R., Norskov, J. K., Barteau, M. A. & Chen, J. G. Role of strain and ligand effects in the modification of the electronic and chemical properties of bimetallic surfaces. Phys. Rev. Lett. 93, 156801 (2004).
Kitchin, J. R. et al. Elucidation of the active surface and origin of the weak metal–hydrogen bond on Ni/Pt(111) bimetallic surfaces: a surface science and density functional theory study. Surf. Sci. 544, 295–308 (2003).
Khan, N. A., Hwu, H. H. & Chen, J. G. Low-temperature hydrodesulfurization of thiophene on Ni/Pt(111) bimetallic surfaces with monolayer Ni coverage. J. Catal. 205, 259–265 (2002).
Sun, Y. M., Sloan, D., Ihm, H. & White, J. M. Electron-induced surface chemistry: production and characterization of NH2 and NH species on Pt(111). J. Vac. Sci. Technol. A 14, 1516–1521 (1996).
Skoplyak, O., Barteau, M. A. & Chen, J. G. Reforming of oxygenates for H2 production: correlating reactivity of ethylene glycol and ethanol on Pt(111) and Ni/Pt(111) with surface d-band center. J. Phys. Chem. B 110, 1686–1694 (2006).
Dietrich, H., Jacobi, K. & Ertl, G. Coverage, lateral order and vibrations of atomic nitrogen on Ru(0001). J. Chem. Phys. 105, 8944–8950 (1996).
Gardin, D. E., Batteas, J. D., Vanhove, M. A. & Somorjai, G. A. Carbon, nitrogen, and sulfur on Ni(111)—formation of complex structures and consequences for molecular decomposition. Surf. Sci. 296, 25–35 (1993).
Kim, Y. K., Morgan, G. A. & Yates, J. T. Site-specific dissociation of N2 on the stepped Ru(109) surface. Surf. Sci. 598, 14–21 (2005).
Dietrich, H., Jacobi, K. & Ertl, G. Decomposition of NH3 on Ru(11(2)–1). Surf. Sci. 352, 138–141 (1996).
Dietrich, H., Jacobi, K. & Ertl, G. Vibrations, coverage, and lateral order of atomic nitrogen and formation of NH3 on Ru(10(1)–0). J. Chem. Phys. 106, 9313–9319 (1997).
Menning, C. A. & Chen, J. G. Thermodynamics and kinetics of oxygen-induced segregation of 3d metals in Pt–3d–Pt(111) and Pt–3d–Pt(100) bimetallic structures. J. Chem. Phys. 128, 174709 (2008).
Menning, C. A. & Chen, J. G. Regenerating Pt–3d–Pt model electrocatalysts through oxidation–reduction cycles monitored at atmospheric pressure. J. Power Sources 195, 3140–3144 (2010).
Lu, S. L. et al. Low temperature hydrogenation of benzene and cyclohexene: a comparative study between gamma-Al2O3 supported PtCo and PtNi bimetallic catalysts. J. Catal. 259, 260–268 (2008).
Humbert, M. P., Murillo, L. E. & Chen, J. G. Rational design of platinum-based bimetallic catalysts with enhanced hydrogenation activity. ChemPhysChem 9, 1262–1264 (2008).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmuller, J. Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized Eigenvalue formalism. Phys. Rev. B 41, 7892–7895 (1990).
Perdew, J. P. et al. Atoms, molecules, solids and surfaces—applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671–6687 (1992).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Pseudopotential Library. Center for Atomic-Scale Materials Design. https://wiki.fysik.dtu.dk/dacapo/Pseudopotential_Library.
Catlett, C. et al. TeraGrid: analysis of organization, system architecture, and middleware enabling new types of applications, in HPC and Grids in Action (Grandinetti, L. ed.) ‘Advances in Parallel Computing’ series (IOS Press, 2007).
Acknowledgements
This research was supported by the Office of Basic Energy Sciences, Department of Energy grants DE-FG02-06ER15795 and DE-FG02-00ER15104. The DFT calculations were performed using the TeraGrid resources provided by the University of Illinois National Center for Supercomputing Applications (NCSA)41.
Author information
Authors and Affiliations
Contributions
D.A.H. and D.G.V. designed and developed the microkinetic models. D.A.H. and J.G.C. designed and developed the UHV experiments. D.A.H. performed and analysed all modelling and experimental work. All authors contributed to writing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Hansgen, D., Vlachos, D. & Chen, J. Using first principles to predict bimetallic catalysts for the ammonia decomposition reaction. Nature Chem 2, 484–489 (2010). https://doi.org/10.1038/nchem.626
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.626
This article is cited by
-
Investigating Periodic Table Interpolation for the Rational Design of Nanoalloy Catalysts for Green Hydrogen Production from Ammonia Decomposition
Catalysis Letters (2024)
-
Direct synthesis of oxalic acid via oxidative CO coupling mediated by a dinuclear hydroxycarbonylcobalt(III) complex
Nature Communications (2023)
-
Dispersed surface Ru ensembles on MgO(111) for catalytic ammonia decomposition
Nature Communications (2023)
-
Preparation of Lanthanum Hexaaluminate Supported Nickel Catalysts for Hydrogen Production by Ammonia Decomposition
Catalysis Letters (2023)
-
Plasma-Catalytic Ammonia Decomposition for Carbon-Free Hydrogen Production Using Low Pressure-Synthesized Mo2N Catalyst
Plasma Chemistry and Plasma Processing (2023)