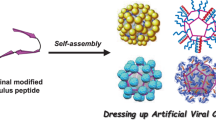
Abstract
Nature offers a vast array of biological building blocks that can be combined with synthetic materials to generate a variety of hierarchical architectures. Viruses are particularly interesting in this respect because of their structure and the possibility of them functioning as scaffolds for the preparation of new biohybrid materials. We report here that cowpea chlorotic mottle virus particles can be assembled into well-defined micrometre-sized objects and then reconverted into individual viruses by application of a short optical stimulus. Assembly is achieved using photosensitive dendrons that bind on the virus surface through multivalent interactions and then act as a molecular glue between the virus particles. Optical triggering induces the controlled decomposition and charge switching of dendrons, which results in the loss of multivalent interactions and the release of virus particles. We demonstrate that the method is not limited to the virus particles alone, but can also be applied to other functional protein cages such as magnetoferritin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mammen, M., Choi, S.-K. & Whitesides, G. M. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 37, 2754–2794 (1998).
Whitesides, G. M. & Grzybowski, B. Self-assembly at all scales. Science 295, 2418–2421 (2002).
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano–bio interface. Nature Mater. 8, 543–557 (2009).
Sarikaya, M. et al. Molecular biomimetics: nanotechnology through biology. Nature Mater. 2, 577–585 (2003).
Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nature Biotechnol. 21, 1171–1178 (2003).
Badjic, J. D. et al. Multivalency and cooperativity in supramolecular chemistry. Acc. Chem. Res. 38, 723–732 (2005).
Mulder, A., Huskens, J. & Reinhoudt, D. N. Multivalency in supramolecular chemistry and nanofabrication. Org. Biomol. Chem. 2, 3409–3424 (2004).
Lee, C. C., MacKay, J. A., Frechet, J. M. J. & Szoka, F. C. Designing dendrimers for biological applications. Nature Biotechnol. 23, 1517–1526 (2005).
Dezzutti, C. S. et al. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 48, 3834–3844 (2004).
Patton, D. L., Cosgrove Sweeney, Y. T., McCarthy, T. D. & Hillier, S. L. Preclinical safety and efficacy assessments of dendrimer-based (SPL7013) microbicide gel formulations in a nonhuman primate model. Antimicrob. Agents Chemother. 50, 1696–1700 (2006).
Rupp, R., Rosenthal, S. L. & Stanberry, L. R. VivaGel™ (SPL7013 Gel): a candidate dendrimer–microbicide for the prevention of HIV and HSV infection. Int. J. Nanomed. 2, 561–566 (2007).
Kostiainen, M. A. & Rosilo, H. Low-molecular-weight dendrons for DNA binding and release by reduction-triggered degradation of multivalent interactions. Chem. Eur. J. 15, 5656–5660 (2009).
Kostiainen, M. A., Smith, D. K. & Ikkala, O. Optically triggered release of DNA from multivalent dendrons by degrading and charge-switching multivalency. Angew. Chem. Int. Ed. 46, 7600–7604 (2007).
Welsh, D. J., Jones, S. P. & Smith, D. K. ‘On–off’ multivalent recognition: degradable dendrons for temporary high-affinity DNA binding. Angew. Chem. Int. Ed. 48, 4047–4051 (2009).
Raja, K. S., Wang, Q. & Finn, M. G. Icosahedral virus particles as polyvalent carbohydrate display platforms. ChemBioChem 4, 1348–1351 (2003).
Tong, G. J., Hsiao, S. C., Carrico, Z. M. & Francis, M. B. Viral capsid DNA aptamer conjugates as multivalent cell-targeting vehicles. J. Am. Chem. Soc. 131, 11174–11178 (2009).
Amir, R. J., Pessah, N., Shamis, M. & Shabat, D. Self-immolative dendrimers. Angew. Chem. Int. Ed. 42, 4494–4499 (2003).
de Groot, F. M. H. et al. ‘Cascade-release dendrimers’ liberate all end groups upon a single triggering event in the dendritic core. Angew. Chem. Int. Ed. 42, 4490–4494 (2003).
de la Escosura, A., Nolte, R. J. M. & Cornelissen, J. J. L. M. Viruses and protein cages as nanocontainers and nanoreactors. J. Mater. Chem. 19, 2274–2278 (2009).
Douglas, T. & Young, M. Viruses: making friends with old foes. Science 312, 873–875 (2006).
Lee, L. A., Niu, Z. & Wang, Q. Viruses and virus-like protein assemblies—chemically programmable nanoscale building blocks. Nano Res. 2, 349–364 (2009).
Uchida, M. et al. Biological containers: protein cages as multifunctional nanoplatforms. Adv. Mater. 19, 1025–1042 (2007).
Douglas, T. & Young, M. Host–guest encapsulation of materials by assembled virus protein cages. Nature 393, 152–155 (1998).
Comellas-Aragones, M. et al. A virus-based single-enzyme nanoreactor. Nature Nanotech. 2, 635–639 (2007).
Davis, H. E., Rosinski, M., Morgan, J. R. & Yarmush, M. L. Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophys. J. 86, 1234–1242 (2004).
Suci, P. A. et al. Assembly of multilayer films incorporating a viral protein cage architecture. Langmuir 22, 8891–8896 (2006).
Sun, J. et al. Core-controlled polymorphism in virus-like particles. Proc. Natl Acad. Sci. USA 104, 1354–1359 (2007).
Yang, L. et al. Self-assembled virus-membrane complexes. Nature Mater. 3, 615–619 (2004).
Li, T. et al. Closed-packed colloidal assemblies from icosahedral plant virus and polymer. Chem. Mater. 21, 1046–1050 (2009).
Li, T. et al. Core/shell biocomposites from the hierarchical assembly of bionanoparticles and polymer. Small 4, 1624–1629 (2008).
Lee, Y. J. et al. Fabricating genetically engineered high-power lithium-ion batteries using multiple virus genes. Science 324, 1051–1055 (2009).
Nam, K. T. et al. Virus-enabled synthesis and assembly of nanowires for lithium ion battery electrodes. Science 312, 885–888 (2006).
Allen, M. et al. Paramagnetic viral nanoparticles as potential high-relaxivity magnetic resonance contrast agents. Magn. Reson. Med. 54, 807–812 (2005).
Gider, S. et al. Classical and quantum magnetic phenomena in natural and artificial ferritin proteins. Science 268, 77–80 (1995).
Kasyutich, O. et al. Small angle X-ray and neutron scattering study of disordered and three-dimensional-ordered magnetic protein arrays. J. Appl. Phys. 105, 07B528 (2009).
Wesley, W., Hsiao, S. C., Carrico, Z. M. & Francis, M. B. Genome-free viral capsids as multivalent carriers for taxol delivery. Angew. Chem. Int. Ed. 48, 9493–9497 (2009).
Tomanin, R. & Scarpa, M. Why do we need new gene therapy viral vectors? Characteristics, limitations and future perspectives of viral vector transduction. Curr. Gene Ther. 4, 357–372 (2004).
Campos, S. K. & Barry, M. A. Current advances and future challenges in adenoviral vector biology and targeting. Curr. Gene Ther. 7, 189–204 (2007).
Jager, L. & Ehrhardt, A. Emerging adenoviral vectors for stable correction of genetic disorders. Curr. Gene Ther. 7, 272–283 (2007).
Speir, J. A. et al. Structures of the native and swollen forms of Cowpea Chlorotic Mottle Virus determined by X-ray crystallography and cryo-electron microscopy. Structure 3, 63–78 (1995).
Kostiainen, M. A., Hardy, J. G. & Smith, D. K. High-affinity multivalent DNA binding by using low-molecular-weight dendrons. Angew. Chem. Int. Ed. 44, 2556–2559 (2005).
Lavelle, L. et al. Phase diagram of self-assembled viral capsid protein polymorphs. J. Phys. Chem. B 113, 3813–3819 (2009).
Zhang, D., Konecny, R., Baker, N. A. & McCammon, J. A. Electrostatic interaction between RNA and protein capsid in Cowpea Chlorotic Mottle Virus simulated by a coarse-grain RNA model and a Monte Carlo approach. Biopolymers 75, 325–337 (2004).
Meldrum, F., Heywood, B. & Mann, S. Magnetoferritin: in vitro synthesis of a novel magnetic protein. Science 257, 522–523 (1992).
Srivastava, S. et al. Integrated magnetic bionanocomposites through nanoparticle-mediated assembly of ferritin. J. Am. Chem. Soc. 129, 11776–11780 (2007).
Perez, J. M. et al. Magnetic relaxation switches capable of sensing molecular interactions. Nature Biotechnol. 20, 816–820 (2002).
Häussler, W., Wilk, A., Gapinski, J. & Patkowski, A. Interparticle correlations due to electrostatic interactions: a small angle X-ray and dynamic light scattering study. I. Apoferritin. J. Chem. Phys. 117, 413–426 (2002).
Josephson, L., Perez, J. M. & Weissleder, R. Magnetic nanosensors for the detection of oligonucleotide sequences. Angew. Chem. Int. Ed. 40, 3204–3206 (2001).
Kasyutich, O., Sarua, A. & Schwarzacher, W. Bioengineered magnetic crystals. J. Phys. D 41, 134022 (2008).
McMillan, R. A. et al. Ordered nanoparticle arrays formed on engineered chaperonin protein templates. Nature Mater. 1, 247–252 (2002).
Verduin, B. J. M. The preparation of CCMV-protein in connection with its association into a spherical particle. FEBS Lett. 45, 50–54 (1974).
Verduin, B. J. M. Degradation of Cowpea Chlorotic Mottle Virus ribonucleic acid in situ. J. Gen. Virol. 39, 131–147 (1978).
Acknowledgements
This work was supported by the Netherlands Organization for Scientific Research (Vidi grant to J.J.L.M.C. and top grant to R.J.M.N.), by the European Research Council (Euryi grant to J.J.L.M.C.) and by the Royal Netherlands Academy of Science (endowed chair to R.J.M.N. and Beijerink award to J.J.L.M.C.). O.K. was supported by the Engineering and Physical Sciences Research Council UK. M.A.K. was supported by the Academy of Finland, Instrumentarium Science Foundation and the Alfred Kordelin Foundation.
Author information
Authors and Affiliations
Contributions
M.A.K., J.J.L.M.C. and R.J.M.N. conceived and designed the experiments. M.A.K. performed the experiments. O.K. contributed to the experiment design and prepared the magnetoferritin. M.A.K., J.J.L.M.C. and R.J.M.N. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1909 kb)
Rights and permissions
About this article
Cite this article
Kostiainen, M., Kasyutich, O., Cornelissen, J. et al. Self-assembly and optically triggered disassembly of hierarchical dendron–virus complexes. Nature Chem 2, 394–399 (2010). https://doi.org/10.1038/nchem.592
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.592
This article is cited by
-
Controlled drug delivery vehicles for cancer treatment and their performance
Signal Transduction and Targeted Therapy (2018)
-
Recent advances in protein-based nanoparticles
Korean Journal of Chemical Engineering (2018)
-
Programmed Self-Assembly of a Biochemical and Magnetic Scaffold to Trigger and Manipulate Microtubule Structures
Scientific Reports (2017)
-
Synthesis of Micelles Guided Magnetite (Fe3O4) Hollow Spheres and their application for AC Magnetic Field Responsive Drug Release
Scientific Reports (2016)
-
Targeted delivery system for cancer cells consist of multiple ligands conjugated genetically modified CCMV capsid on doxorubicin GNPs complex
Scientific Reports (2016)