Abstract
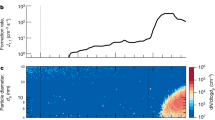
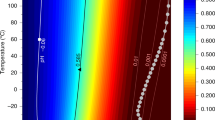
Polar stratospheric clouds (PSCs) are extremely efficient at catalysing the transformation of photostable chlorine reservoirs into photolabile species, which are actively involved in springtime ozone-depletion events. Why PSCs are such efficient catalysts, however, is not well understood. Here, we investigate the freezing behaviour of ternary HNO3–H2SO4–H2O droplets of micrometric size, which form type II PSC ice particles. We show that on freezing, a phase separation into pure ice and a residual solution coating occurs; this coating does not freeze but transforms into glass below ∼150 K. We find that the coating, which is thicker around young ice crystals, can still be approximately 30 nm around older ice crystals of diameter about 10 µm. These results affect our understanding of PSC microphysics and chemistry and suggest that chlorine-activation reactions are better studied on supercooled HNO3–H2SO4–H2O solutions rather than on a pure ice surface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Solomon, S., Garcia, R. R., Rowland, F. S. & Wuebbles, D. J. On the depletion of Antarctic ozone. Nature 321, 755–758 (1986).
Molina M. J., Tso, T. L., Molina, L. T. & Wang, F. C. Y. Antarctic stratospheric chemistry of chlorine nitrate, hydrogen chloride, and ice: release of active chlorine. Science 238, 1253–1257 (1987).
Molina, M. J. in The Chemistry of the Atmosphere: The Impact of Global Change (ed. Calvet, J. G.) 27–38 (Blackwell, 1994).
Molina, M. J., Molina, L. T. & Golden, D. M. Environmental chemistry (gas and gas–solid interactions): the role of physical chemistry. J. Phys. Chem. 100, 12888–12896 (1996).
Prenni, A. J. & Tolbert, M. A. Studies of polar stratospheric cloud formation. Acc. Chem. Res. 34, 545–553 (2001).
Zondlo, M. A., Barone, S. B. & Tolbert, M. A. Condensed-phase products in heterogeneous reactions: N2O5, ClONO2, and HNO3 reacting on ice films at 185 K. J. Phys. Chem. A 102, 5735–5748 (1998).
Zondlo, M. A., Hudson, P. K., Prenni, A. J. & Tolbert, M. A. Chemistry and microphysics of polar stratospheric clouds and cirrus clouds. Ann. Rev. Phys. Chem. 51, 473–499 (2000).
Chang, H.-Y. A., Koop, T., Molina, L. T., & Molina, M. J. Phase transitions in emulsified HNO3/H2O and HNO3/H2SO4/H2O solutions. J. Phys. Chem. A 103, 2673–2679 (1999).
Buesnel, R., Hillier, I. H. & Masters, A. J. Molecular dynamics simulation of the ionization of hydrogen chloride in water clusters using a quantum mechanical potential. Chem. Phys. Lett. 247, 391–394 (1995).
Pursell, C. J., Zaidi, M., Thompson, A., Franser-Caston, C. & Vela, E. Acid–base chemistry on crystalline ice: HCl + NH3 . J. Phys. Chem. A 104, 552–556 (2000).
McNeil, V. F., Loerting, T., Geiger, F. M., Trout, B. L. & Molina, M. J. Hydrogen chloride-induced surface disordering on ice. Proc. Natl Acad. Sci. USA 103, 9422–9427 (2006).
McNeill, V. F. et al. Interaction of hydrogen chloride with ice surfaces: the effects of grain size, surface roughness, and surface disorder. J. Phys. Chem. A 111, 6274–6284 (2007).
Fluckiger, B., Chaix, L. & Rossi, M. J. Properties of the HCl/ice, HBr/ice, and H2O/ice interface at stratospheric temperatures (200 K) and its importance for atmospheric heterogeneous reactions. J. Phys. Chem. A 104, 11739–11750 (2000).
Bogdan, A., Kulmala, M., MacKenzie, A. R. & Laaksonen, A. Study of finely divided aqueous systems as an aid to understanding the surface chemistry of polar stratospheric clouds: case of HCl/H2O and HNO3/HCl/H2O systems. J. Geophys. Res. 108, 4303, doi:10.1029/2002JD002606 (2003).
Oppliger, R., Allanic, A. & Rossi, M. J. Real-time kinetics of the uptake of ClONO2 on ice and in the presence of HCl in the temperature range 160 K ≤ T ≤ 200 K. J. Phys. Chem. A 101, 1903–1911 (1997).
Gertner, B. J. & Hynes, J. T. Molecular dynamic simulation of hydrochloric acid ionization at the surface of stratospheric ice. Science 271, 1563–1565 (1996).
Wofsy, S. C., Molina, M. J., Salawitch, R. J., Fox, L. E. & McElroy, M. B. Interactions between HCl, NOx, and H2O ice in the Antarctic stratosphere: implication for ozone. J. Geophys. Res. 93, 2442–2450 (1988).
Angell, C. A. The old problems of glass and the glass transition, and the many new twists. Proc. Natl Acad. Sci. USA 92, 6675–6682 (1995).
Angell, C. A. Formation of glasses from liquids and biopolymers. Science 267, 1924–1935 (1995).
Debenedetti, P. G. & Stillinger, F. H. Supercooled liquids and the glass transition. Nature 410, 259–267 (2001).
Bogdan, A., Molina, M. J., Sassen, K. & Kulmala, M. Formation of low-temperature cirrus from H2SO4/H2O aerosol droplets. J. Phys. Chem. A 110, 12541–12542 (2006).
Bogdan, A. & Molina, M. J. Why does large relative humidity with respect to ice persist in cirrus ice clouds? J. Phys. Chem. A 113, 14123–14130 (2009). doi: 10.1021/jp9063609.
Bogdan, A. Reversible formation of glassy water in slowly cooling diluted drops. J. Phys. Chem. B 110, 12205–12206 (2006).
Delval, C. & Rossi, M. J. Influence of monolayer amounts of HNO3 on the evaporation rate of H2O over ice in the range 179 to 208 K: a quartz crystal microbalance study. J. Phys. Chem. A 109, 7151–7165 (2005).
Beyer, K. D., Hansen, A. R. & Raddatz, N. Experimental determination of the H2SO4/HNO3/H2O phase diagram in regions of stratospheric importance. J. Phys. Chem. A 108, 770–780 (2004).
Beyer, K. D., Hansen, A. R. & Poston, M. The search for sulfuric acid octahydrate: experimental evidence. J. Phys. Chem. A 107, 2025–2932 (2003).
Gable, C. M., Betz, H. F. & Maron, S. H. Phase equilibria of the system sulfur trioxide–water. J. Am. Chem. Soc. 72, 1445–1448 (1950).
Carslaw, K. S. et al. Particle microphysics and chemistry in remotely observed mountain polar stratospheric clouds. J. Geophys. Res. 103, 5785–5796 (1998).
Macfarlane, D. R. Continuous cooling (CT) diagrams and critical cooling rates: a direct method of calculations using the concept of additivity. J. Non-Cryst. Solids 53, 61–72 (1982).
Lawson, R. P. et al. Aircraft measurements of microphysical properties of subvisible cirrus in the tropical tropopause layer. Atmos. Chem. Phys. 8, 1609–1620 (2008).
Deshler, T., Peter, T., Müller, R. & Crutzen, P. The lifetime of leewave-induced ice particles in the Arctic stratosphere: I. Balloonborne observations. Geophys. Res. Lett. 21, 1327–1330 (1994).
Acknowledgements
We thank E. Bertel, S.C. Wofsy and M.B. McElroy for discussions. A.B. thanks the Physical and Chemical Departments of the University of Helsinki for providing facilities to perform the experiments. We are grateful for financial support from the European Research Council (Starting Grant SULIWA) and the Austrian Science Fund (START award Y391).
Author information
Authors and Affiliations
Contributions
A.B. designed the study, performed measurements and calculations, collected and analysed data, and wrote the manuscript. M.J.M. designed the study, analysed data, and wrote and corrected the manuscript. H.T., E.M. and T.L. analysed data, and wrote and corrected the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bogdan, A., Molina, M., Tenhu, H. et al. Formation of mixed-phase particles during the freezing of polar stratospheric ice clouds. Nature Chem 2, 197–201 (2010). https://doi.org/10.1038/nchem.540
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.540
This article is cited by
-
The decisive role of free water in determining homogenous ice nucleation behavior of aqueous solutions
Scientific Reports (2016)
-
Visualization of Freezing Process in situ upon Cooling and Warming of Aqueous Solutions
Scientific Reports (2014)