Abstract
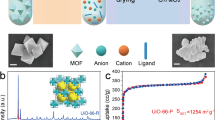
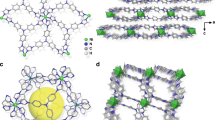
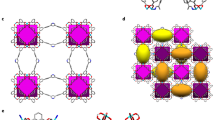
The selective capture of Cs+ from solution is relevant to the remediation of nuclear waste and remains a significant challenge. Here we describe a new framework composed of [(CH3)2NH2]+ and [Ga2Sb2S7]2− layers, which are perforated with holes. Shape selectivity couples with framework flexibility, allowing the compound to respond to the ion-exchange process. The size, shape and flexibility of the holes allow Cs+ ions in an aqueous solution to selectively pass through and enter the material via an ion-exchange process. Following capture, the structure dynamically closes its holes in a manner reminiscent of a Venus flytrap, which prevents the Cs+ ions from leaching out. This process has useful implications in the separation science of Cs as it relates to the clean-up of nuclear waste. The dynamic response we describe here provides important insights for designing new materials for the selective removal of difficult-to-capture ions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Li, H., Laine, A. & O'Keeffe, M. Supertetrahedral sulfide crystals with giant cavities and channels. Science 283, 1145–1147 (1999).
MacLachlan, M. J., Coombs, N. & Ozin, G. A. Non-aqueous supramolecular assembly of mesostructured metal germanium sulfides from Ge4S104− clusters. Nature 397, 681–684 (1999).
Bag, S., Trikalitis, P. N., Chupas, P. J. & Kanatzidis, M. G. Porous semiconducting gels and aerogels from chalcogenide clusters. Science 317, 490–493 (2007).
Zheng, N., Bu, X. & Feng, P. Synthetic design of crystalline inorganic chalcogenides exhibiting fast-ion conductivity. Nature 426, 428–432 (2003).
Zheng, N., Bu, X. & Feng, P. Pentasupertetrahedral clusters as building blocks for a three-dimensional sulfide superlattice. Angew. Chem. Int. Ed. Engl. 43, 4753–4755 (2004).
Manos, M. J., Iyer, R. G., Quarez, E., Liao, J. H. & Kanatzidis, M. G. {Sn[Zn4Sn4S17]}6−: A robust open framework based on metal-linked penta-supertetrahedral [Zn4Sn4S17]10− clusters with ion-exchange properties. Angew. Chem. 44, 3552–3555 (2005).
Spetzler, V., Riujberk, H., Näther, C. & Bensch, W. Novel copper(i)-thioantimonates(iii): solvothermal synthesis, crystal structures, thermal stability and magnetic properties of (C2N2H10)0.5Cu2SbS3, (C3N2H12)0.5Cu2SbS3 and (C4N2H14)0.5Cu2SbS3 . Z. Anorg. Allg. Chem. 630, 142–148 (2004).
Hanko J. A. & Kanatzidis M. G. A three-dimensional framework with accessible nanopores: RbCuSb2Se4·H2O. Angew. Chem. Int. Ed. 37, 342–344 (1998).
Parise, J. B. An antimony sulfide with a 2-dimensional, intersecting system of channels. Science 251, 293–294 (1991).
Vaqueiro, P., Chippindale, A. M., Cowley, A. R. & Powell, A. V. Templated synthesis of the novel layered silver − antimony sulfides [H3NCH2CH2NH2][Ag2SbS3] and [H3NCH2CH2NH2]2[Ag5Sb3S8]. Inorg. Chem. 42, 7846–7851 (2003).
van den Berg, A. W. C., Bromley, S. T., Ramsahye, N. & Maschmeyer, T. Diffusion of molecular hydrogen through porous materials: the importance of framework flexibility. J. Phys. Chem. B 108, 5088–5094 (2004).
Fletcher, A. J., Thomas, K. M. & Rosseinsky, M. J. Flexibility in metal-organic framework materials: Impact on sorption properties. J. Solid State Chem. 178, 2491–2510 (2005).
Trikalitis, P. N., Ding, N., Malliakas, C., Billinge, S. J. L. & Kanatzidis, M. G. Mesostructured selenides with cubic MCM-48 type symmetry: Large framework elasticity and uncommon resiliency to strong acids. J. Am. Chem. Soc. 126, 15326–15327 (2004).
Manos, M. J., Chrissafis K. & Kanatzidis M. G. Unique pore selectivity for Cs+ and exceptionally high NH4+ exchange capacity of the chalcogenide material K6Sn[Zn4Sn4S17]. J. Am. Chem. Soc. 128, 8875–8883 (2006).
Venkatesan K. A., Sukumaran V., Antony M. P. & Srinivasan, T. G. Studies on the feasibility of using crystalline silicotitanates for the separation of cesium-137 from fast reactor high-level liquid waste. J. Rad. Nucl. Chem. 280, 129–136, (2009).
Bortun, A. I., Bortun, L. N., Poojary, D. M., Xiang, O. & Clearfield, A. Synthesis, characterization, and ion exchange behavior of a framework potassium titanium trisilicate K2TiSi3O9·H2O and its protonated phases. Chem. Mater. 12, 294–305 (2000).
Gu, B. et al. The effect of H+ irradiation on the Cs-ion exchange capacity of zeolite-NaY. J. Mater. Chem. 10, 2610–2616 (2000).
Möller, T. et al. Uptake of 85Sr, 134Cs and 57Co by antimony silicates doped with Ti4+, Nb5+, Mo6+ and W6+. J. Mater. Chem. 11, 1526–1532 (2001).
Celestian, A. & Clearfield, A. The origin of ion-exchange selectivity in a porous framework titanium silicate. J. Mater. Chem. 17, 4839–4842 (2007).
Korzenski, M. B. & Kolis, J. W. Structural, magnetic, and ion-exchange properties of a new layered alkaline/alkaline earth iron phosphate: NaBaFe4(HPO4)3(PO4)3·H2O. Inorg. Chem. 39, 5663–5668 (2000).
Ok, K. M., Baek, J., Halasyamani, P. S. & O'Hare, D. New layered uranium phosphate fluorides: syntheses, structures, characterizations, and ion-exchange properties of A(UO2)F(HPO4)·xH2O (A = Cs+, Rb+, K+; x = 0 − 1). Inorg. Chem. 45, 10207–10214 (2006).
Manos, M. J., Ding, N. & Kanatzidis, M. G. Layered metal sulfides: exceptionally selective agents for radioactive strontium removal Proc. Natl Acad. Sci. USA 105, 3696–3699 (2008).
Manos, M. J. & Kanatzidis, M. G. Highly efficient and rapid Cs+ uptake by the layered metal sulfide K2xMnxSn3−xS6 (KMS-1). J. Am. Chem. Soc. 131, 6599–6607 (2009).
Gash, A. E. et al. Efficient recovery of elemental mercury from Hg(ii)-contaminated aqueous media using a redox-recyclable ion-exchange material. Environ. Sci. Technol. 32, 1007–1012 (1998).
Behrens, E. A., Sylvester, P. & Clearfield, A. Assessment of a sodium nonatitanate and pharmacosiderite-type ion exchangers for strontium and cesium removal from DOE waste stimulants. Environ. Sci. Technol. 32, 101–107 (1998).
Acknowledgements
This research was supported by National Science Foundation (DMR-0801855). This work made use of the ICP-OES (supported by National Science Foundation) at the Integrated Molecular Structure Education and Research Center (IMSERC) at Northwestern University.
Author information
Authors and Affiliations
Contributions
N.D. and M.G.K. conceived and designed the experiments, N.D. performed the experiments, N.D. and M.G.K. analysed the data and co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 296 kb)
Supplementary information
Crystallographic data for compound I (CIF 11 kb)
Supplementary information
Crystallographic data for compound II (CIF 19 kb)
Rights and permissions
About this article
Cite this article
Ding, N., Kanatzidis, M. Selective incarceration of caesium ions by Venus flytrap action of a flexible framework sulfide. Nature Chem 2, 187–191 (2010). https://doi.org/10.1038/nchem.519
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.519
This article is cited by
-
Effective removal of cesium ions by using PAN/PANI blend nanofibers prepared by electrospinning method
Journal of Radioanalytical and Nuclear Chemistry (2024)
-
Highly selective cesium(I) capture under acidic conditions by a layered sulfide
Nature Communications (2022)
-
Facile Synthesis of Porous Polymer Using Biomass Polyphenol Source for Highly Efficient Separation of Cs+ from Aqueous Solution
Scientific Reports (2020)
-
Stimuli-Responsive Biopolymers: An Inspiration for Synthetic Smart Materials and Their Applications in Self-Controlled Catalysis
Journal of Inorganic and Organometallic Polymers and Materials (2020)
-
Removal of cesium ions from aqueous solutions using various separation technologies
Reviews in Environmental Science and Bio/Technology (2019)