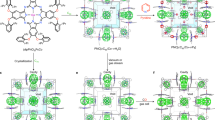
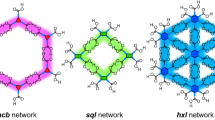
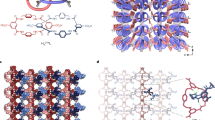
Abstract
Most synthetic materials that show molecular-scale porosity consist of one-, two- or three-dimensional networks. Porous metal-organic frameworks in particular have attracted a lot of recent attention. By contrast, discrete molecules tend to pack efficiently in the solid state, leaving as little empty space as possible, which leads to non-porous materials. This Perspective discusses recent developments with discrete organic molecules that are porous in the solid state. Such molecules, which may be either crystalline or amorphous, can be categorized as either intrinsically porous (containing permanent covalent cavities) or extrinsically porous (inefficiently packed). We focus on the possible advantages of organic molecules over inorganic or hybrid systems in terms of molecular solubility, choice of components and functionalities, and structural mobility and responsiveness in non-covalent extended solids. We also highlight the potential for 'undiscovered' porous systems among the large number of cage-like organic molecules that are already known.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bradshaw, D., Claridge, J. B., Cussen, E. J., Prior, T. J. & Rosseinsky, M. J. Design, chirality, and flexibility in nanoporous molecule-based materials. Acc. Chem. Res. 38, 273–282 (2005).
Horike, S., Shimomura, S. & Kitagawa, S. Soft porous crystals. Nature Chem. 1, 695–704 (2009).
Wright, P. A. Microporous framework solids (Royal Society of Chemistry, 2007).
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003).
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Jiang, J. X. et al. Conjugated microporous poly(aryleneethynylene) networks. Angew. Chem. Int. Ed. 46, 8574–8578 (2007).
Barbour, L. J. Crystal porosity and the burden of proof. Chem. Commun. 1163–1168 (2006).
Atwood, J. L., Barbour, L. J. & Jerga, A. Storage of methane and Freon by interstitial van der Waals confinement. Science 296, 2367–2369 (2002).
Atwood, J. L., Barbour, L. J., Jerga, A. & Schottel, B. L. Guest transport in a nonporous organic solid via dynamic van der Waals cooperativity. Science 298, 1000–1002 (2002).
Sudik, A. C. et al. Design, synthesis, structure, and gas (N2, Ar, CO2, CH4, and H2) sorption properties of porous metal-organic tetrahedral and heterocuboidal polyhedra. J. Am. Chem. Soc. 127, 7110–7118 (2005).
Kowalczyk, P., Brualla, L., Zywocinski, A. & Bhatia, S. K. Single-walled carbon nanotubes: Efficient nanomaterials for separation and on-board vehicle storage of hydrogen and methane mixture at room temperature? J. Phys. Chem. C 111, 5250–5257 (2007).
Ben, T. et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 48, 9457–9460 (2009).
Furukawa, H. et al. Ultrahigh porosity in metal-organic frameworks. Science 329, 424–428 (2010).
Budd, P. M. et al. Polymers of intrinsic microporosity (PIMs): robust, solution-processable, organic nanoporous materials. Chem. Commun. 230–231 (2004).
Tozawa, T. et al. Porous organic cages. Nature Mater. 8, 973–978 (2009).
Lim, S. et al. Cucurbit[6]uril: organic molecular porous material with permanent porosity, exceptional stability, and acetylene sorption properties. Angew. Chem. Int. Ed. 47, 3352–3355 (2008).
O'Reilly, N., Giri, N. & James, S. L. Porous liquids. Chem. Eur. J. 13, 3020–3025 (2007).
Barrer, R. M. & Shanson, V. H. Dianin's compound as a zeolitic sorbent. J. Chem. Soc. Chem. Commun. 333–334 (1976).
Imashiro, F., Yoshimura, M. & Fujiwara, T. 'Guest-free' Dianin's compound. Acta Crystallogr. C 54, 1357–1360 (1998).
Sozzani, P., Bracco, S., Comotti, A., Ferretti, L. & Simonutti, R. Methane and carbon dioxide storage in a porous van der Waals crystal. Angew. Chem. Int. Ed. 44, 1816–1820 (2005).
Gorbitz, C. H. & Gundersen, E. L-Valyl-L-alanine. Acta Crystallogr. C 52, 1764–1767 (1996).
Soldatov, D. V., Moudrakovski, I. L., Grachev, E. V. & Ripmeester, J. A. Micropores in crystalline dipeptides as seen from the crystal structure, He pycnometry, and 129Xe NMR spectroscopy. J. Am. Chem. Soc. 128, 6737–6744 (2006).
Comotti, A., Bracco, S., Distefano, G. & Sozzani, P. Methane, carbon dioxide and hydrogen storage in nanoporous dipeptide-based materials. Chem. Commun. 284–286 (2009).
Msayib, K. J. et al. Nitrogen and hydrogen adsorption by an organic microporous crystal. Angew. Chem. Int. Ed. 48, 3273–3277 (2009).
Goldberg, I. Crystal engineering of nanoporous architectures and chiral porphyrin assemblies. CrystEngComm 10, 637–645 (2008).
Hosseini, M. W. Molecular tectonics: From simple tectons to complex molecular networks. Acc. Chem. Res. 38, 313–323 (2005).
Wuest, J. D. Engineering crystals by the strategy of molecular tectonics. Chem. Commun. 5830–5837 (2005).
Day, G. M. et al. Significant progress in predicting the crystal structures of small organic molecules — a report on the fourth blind test. Acta Crystallogr. B 65, 107–125 (2009).
Rodriguez-Albelo, L. M. et al. Zeolitic polyoxometalate-based metal-organic frameworks (Z-POMOFs): Computational evaluation of hypothetical polymorphs and the successful targeted synthesis of the redox-active Z-POMOF1. J. Am. Chem. Soc. 131, 16078–16087 (2009).
Dalgarno, S. J., Thallapally, P. K., Barbour, L. J. & Atwood, J. L. Engineering void space in organic van der Waals crystals: calixarenes lead the way. Chem. Soc. Rev. 36, 236–245 (2007).
Thallapally, P. K. et al. Carbon dioxide capture in a self-assembled organic nanochannels. Chem. Mater. 19, 3355–3357 (2007).
Tedesco, C. et al. Methane adsorption in a supramolecular organic zeolite. Chem. Eur. J. 16, 2371–2374 (2010).
Dewal, M. B. et al. Absorption properties of a porous organic crystalline apohost formed by a self-assembled bis-urea macrocycle. Chem. Mater. 18, 4855–4864 (2006).
Xu, Y. et al. Thermal reaction of a columnar assembled diacetylene macrocycle. J. Am. Chem. Soc. 132, 5334–5335 (2010).
Cram, D. J. Cavitands — organic hosts with enforced cavities. Science 219, 1177–1183 (1983).
Collet, A. Cyclotriverarylenes and cryptophanes. Tetrahedron 43, 5725–5759 (1987).
Leontiev, A. V. & Rudkevich, D. M. Encapsulation of gases in the solid state. Chem. Commun. 1468–1469 (2004).
Hof, F., Craig, S. L., Nuckolls, C. & Rebek, J. Molecular encapsulation. Angew. Chem. Int. Ed. 41, 1488–1508 (2002).
Mastalerz, M. Shape-persistent organic cage compounds by dynamic covalent bond formation. Angew. Chem. Int. Ed. 49, 5042–5053 (2010).
Hasell, T. et al. Triply interlocked covalent organic cages. Nature Chem. 2, 750–755 (2010).
Bezzu, C. G., Helliwell, M., Warren, J. E., Allan, D. R. & McKeown, N. B. Heme-like coordination chemistry within nanoporous molecular crystals. Science 327, 1627–1630 (2010).
Rowan, S. J., Cantrill, S. J., Cousins, G. R. L., Sanders, J. K. M. & Stoddart, J. F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 41, 898–952 (2002).
McKeown, N. B. & Budd, P. M. Polymers of intrinsic microporosity (PIMs): organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 35, 675–683 (2006).
Ghanem, B. S. et al. Synthesis, characterization, and gas permeation properties of a novel group of polymers with intrinsic microporosity: PIM-polyimides. Macromolecules 42, 7881–7888 (2009).
Weber, J., Su, O., Antonietti, M. & Thomas, A. Exploring polymers of intrinsic microporosity — microporous, soluble polyamide and polyimide. Macromol. Rapid Commun. 28, 1871–1876 (2007).
Carta, M., Msayib, K. J., Budd, P. M. & McKeown, N. B. Novel spirobisindanes for use as precursors to polymers of intrinsic microporosity. Org. Lett. 10, 2641–2643 (2008).
Tian, J., Thallapally, P. K., Dalgarno, S. J., McGrail, P. B. & Atwood, J. L. Amorphous molecular organic solids for gas adsorption. Angew. Chem. Int. Ed. 48, 5492–5495 (2009).
Kudo, H. et al. Molecular waterwheel (Noria) from a simple condensation of resorcinol and an alkanedial. Angew. Chem. Int. Ed. 45, 7948–7952 (2006).
Xu, D. & Warmuth, R. Edge-directed dynamic covalent synthesis of a chiral nanocube. J. Am. Chem. Soc. 130, 7520–7521 (2008).
Murakami, Y., Kikuchi, J. & Hirayama, T. Cubic azaparacyclophane. Chem. Lett. 16, 161–164 (1987).
Mastalerz, M. One-pot synthesis of a shape-persistent endo-functionalised nano-sized adamantoid compound. Chem. Commun. 4756–4758 (2008).
Zhang, C. & Chen, C.-F. Synthesis and structure of a triptycene-based nanosized molecular cage. J. Org. Chem. 72, 9339–9341 (2007).
Acknowledgements
We thank the Engineering and Physical Sciences Research Council (EPSRC) for supporting this work (EP/H000925/1). A.I.C. holds a Royal Society Wolfson Research Merit Award. A.T. is a Royal Society University Research Fellow. We thank T. Mitra for assistance with the preparation of Fig. 1b.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holst, J., Trewin, A. & Cooper, A. Porous organic molecules. Nature Chem 2, 915–920 (2010). https://doi.org/10.1038/nchem.873
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.873
This article is cited by
-
Polyimide covalent organic frameworks as efficient solid-state Li+ electrolytes
Science China Chemistry (2024)
-
Multiple yet switchable hydrogen-bonded organic frameworks with white-light emission
Nature Communications (2022)
-
Pre-arranged building block approach for the orthogonal synthesis of an unfolded tetrameric organic–inorganic phosphazane macrocycle
Communications Chemistry (2022)
-
Inclusion of organic molecule guests by sulfinyl bridged bis-salicylic acid-type open-chain host with flexible change of crystal structure
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2022)
-
Porous solid inspired hyper-crosslinked polymer liquids with highly efficient regeneration for gas purification
Science China Materials (2022)