Abstract
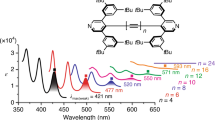
Carbyne is an allotrope of carbon composed of sp-hybridized carbon atoms. Although its formation in the laboratory is suggested, no well-defined sample is described. Interest in carbyne and its potential properties remains intense because of, at least in part, technological breakthroughs offered by other carbon allotropes, such as fullerenes, carbon nanotubes and graphene. Here, we describe the synthesis of a series of conjugated polyynes as models for carbyne. The longest of the series consists of 44 contiguous acetylenic carbons, and it maintains a framework clearly composed of alternating single and triple bonds. Spectroscopic analyses for these polyynes reveal a distinct trend towards a finite gap between the highest occupied molecular orbital and the lowest unoccupied molecular orbital for carbyne, which is estimated to be ∼485 nm (∼2.56 eV). Even the longest members of this series of polyynes are not particularly sensitive to light, moisture or oxygen, and they can be handled and characterized under normal laboratory conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mannion, A. M. Carbon and its Domestication (Springer, 2006).
Pierson, H. O. Handbook of Carbon, Graphite, Diamond and Fullerenes – Properties, Processing and Applications (William Andrew Publishing/Noyes, 1993).
Falcao, E. H. L. & Wudl, F. Carbon allotropes: beyond graphite and diamond. J. Chem. Technol. Biotechnol. 82, 524–531 (2007).
Webster, A. Carbyne as a possible constituent of the interstellar dust. Mon. Not. R. Astron. Soc. 192, 7–9 (1980).
Hayatsu, R., Scott, R. G., Studier, M. H., Lewis, R. S. & Anders, E. Carbynes in meteorites: detection, low-temperature origin, and implications for interstellar molecules. Science 209, 1515–1518 (1980).
El Goresy, A. & Donnay, G. A new allotropic form of carbon from the Ries crater. Science 161, 363–364 (1968).
Lagow, R. J. et al. Synthesis of linear acetylenic carbon: the ‘sp’ carbon allotrope. Science 267, 362–367 (1995).
Cataldo, F. Polyynes: Synthesis, Properties and Applications (Taylor & Francis, 2005).
Chalifoux, W. A. & Tykwinski, R. R. Synthesis of extended polyynes: toward carbyne. C. R. Chim. 12, 341–358 (2009).
Eastmond, R., Johnson, T. R. & Walton, D. R. M. Silylation as a protective method for terminal alkynes in oxidative couplings. Tetrahedron 28, 4601–4616 (1972).
Zheng, Q. et al. A synthetic breakthrough into an unanticipated stability regime: a series of isolable complexes in which C6, C8, C10, C12, C16, C20, C24, and C28 polyynediyl chains span two platinum atoms. Chem. Eur. J. 12, 6486–6505 (2006).
Khuong, T.-A. V., Zepeda, G., Ruiz, R., Khan, S. I. & Garcia-Garibay, M. A. Molecular compasses and gyroscopes: engineering molecular crystals with fast internal rotation. Cryst. Growth Des. 4, 15–18 (2004).
Jahnke, E. & Tykwinski, R. R. The Fritsch–Buttenberg–Wiechell rearrangement: modern applications for an old reaction. Chem. Commun. 46, 3235–3249 (2010).
Eglinton, G. & Galbraith, A. R. Cyclic diynes. Chem. Ind. (London) 737–738 (1956).
Siemsen, P., Livingston, R. C. & Diederich, F. Acetylenic coupling: a powerful tool in molecular construction. Angew. Chem. Int. Ed. 39, 2633–2657 (2000).
Haley, M. M. et al. One-pot desilylation/dimerization of ethynyl- and butadiynyltrimethylsilanes. Synthesis of tetrayne-linked dehydrobenzoannulenes. Tetrahedron Lett. 38, 7483–7486 (1997).
Wang, C., Batsanov, A. S., West, K. & Bryce, M. R. Synthesis and crystal structures of isolable terminal aryl hexatriyne and octatetrayne derivatives: Ar–(C≡C)nH (n=3, 4). Org. Lett. 10, 3069–3072 (2008).
Hay, A. S. Oxidative coupling of acetylenes II. J. Org. Chem. 27, 3320–3321 (1962).
Schermann, G., Grösser, T., Hampel, F. & Hirsch, A. Dicyanopolyynes: a homologous series of end-capped linear sp carbon. Chem. Eur. J. 3, 1105–1112 (1997).
Eisler, S. et al. Polyynes as a model for carbyne: synthesis, physical properties, and nonlinear optical response. J. Am. Chem. Soc. 127, 2666–2676 (2005).
Gibtner, T., Hampel, F., Gisselbrecht, J.-P. & Hirsch, A. End-cap stabilized oligoynes: model compounds for the linear sp carbon allotrope carbyne. Chem. Eur. J. 8, 408–432 (2002).
Mohr, W., Stahl, J., Hampel, F. & Gladysz, J. A. Synthesis, structure, and reactivity of sp carbon chains with bis(phosphine) pentafluorophenylplatinum endgroups: butadiynediyl (C4) through hexadecaoctaynediyl (C16) bridges, and beyond. Chem. Eur. J. 9, 3324–3340 (2003).
Meier, H., Stalmach, U. & Kolshorn, H. Effective conjugation length and UV/vis spectra of oligomers. Acta Polym. 48, 379–384 (1997).
Martin, R. E. & Diederich, F. Linear monodisperse π-conjugated oligomers: model compounds for polymers and more. Angew. Chem. Int. Ed. 38, 1350–1377 (1999).
Hoffmann, R. How chemistry and physics meet in the solid state. Angew. Chem. Int. Ed. 26, 846–878 (1987).
Chalifoux, W. A., McDonald, R., Ferguson, M. J. & Tykwinski, R. R. tert-Butyl-end-capped polyynes: crystallographic evidence of reduced bond-length alternation. Angew. Chem. Int. Ed. 48, 7915–7919 (2009).
Szafert, S. & Gladysz, J. A. Carbon in one dimension: structural analysis of the higher conjugated polyynes. Chem. Rev. 103, 4175–4205 (2003).
Szafert, S. & Gladysz, J. A. Update 1 of: Carbon in one dimension: structural analysis of the higher conjugated polyynes. Chem. Rev. 106, PR1–PR33 (2006).
Acknowledgements
This work was supported by the University of Alberta and the Natural Sciences and Engineering Research Council of Canada (NSERC) through the Discovery Grant program. W.A.C. thanks the NSERC (Postgraduate Scholarship-D) and the Alberta Ingenuity Fund for scholarship support. We also thank F. Marsiglio for discussions and M. Ferguson and R. McDonald for solving the X-ray structures for 4f and 1b, respectively.
Author information
Authors and Affiliations
Contributions
W.A.C. designed the experiments, and performed the syntheses, characterization and property studies. W.A.C. and R.R.T co-wrote the paper. R.R.T conceived the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 3724 kb)
Supplementary information
Crystallographic data for compound 1b (CIF 29 kb)
Supplementary information
Crystallographic data for compound 4f (CIF 33 kb)
Rights and permissions
About this article
Cite this article
Chalifoux, W., Tykwinski, R. Synthesis of polyynes to model the sp-carbon allotrope carbyne. Nature Chem 2, 967–971 (2010). https://doi.org/10.1038/nchem.828
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.828
This article is cited by
-
Masked alkynes for synthesis of threaded carbon chains
Nature Chemistry (2024)
-
Thermoelectric properties of topological chains coupled to a quantum dot
Scientific Reports (2023)
-
The preparation of whole sp-C composed alkyne rich carbon materials
Nano Research (2023)
-
Electron-phonon coupling and vibrational properties of size-selected linear carbon chains by resonance Raman scattering
Nature Communications (2022)
-
Structural and electronic properties of polyyne and cumulene chains with phenylene as central aromatic group: a density functional theory study
Structural Chemistry (2022)